Structural considerations for functional anti-EGFR × anti-CD3 bispecific diabodies in light of domain order and binding affinity
- PMID: 29568402
- PMCID: PMC5862623
- DOI: 10.18632/oncotarget.24490
Structural considerations for functional anti-EGFR × anti-CD3 bispecific diabodies in light of domain order and binding affinity
Abstract
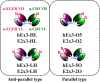
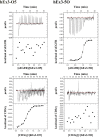
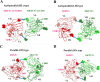
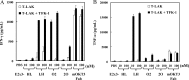
We previously reported a functional humanized bispecific diabody (bsDb) that targeted EGFR and CD3 (hEx3-Db) and enhancement of its cytotoxicity by rearranging the domain order in the V domain. Here, we further dissected the effect of domain order in bsDbs on their cross-linking ability and binding kinetics to elucidate general rules regarding the design of functional bsDbs. Using Ex3-Db as a model system, we first classified the four possible domain orders as anti-parallel (where both chimeric single-chain components are variable heavy domain (VH)-variable light domain (VL) or VL-VH order) and parallel types (both chimeric single-chain components are mixed with VH-VL and VL-VH order). Although anti-parallel Ex3-Dbs could cross-link the soluble target antigens, their cross-linking ability between soluble targets had no correlation with their growth inhibitory effects. In contrast, the binding affinity of one of the two constructs with a parallel-arrangement V domain was particularly low, and structural modeling supported this phenomenon. Similar results were observed with E2x3-Dbs, in which the V region of the anti-EGFR antibody clone in hEx3 was replaced with that of another anti-EGFR clone. Only anti-parallel types showed affinity-dependent cancer inhibitory effects in each molecule, and E2x3-LH (both components in VL-VH order) showed the most intense anti-tumor activity in vitro and in vivo. Our results showed that, in addition to rearranging the domain order of bsDbs, increasing their binding affinity may be an ideal strategy for enhancing the cytotoxicity of anti-parallel constructs and that E2x3-LH is particularly attractive as a candidate next-generation anti-cancer drug.
Keywords: CD3; EGFR; bispecific diabody; cancer immunotherapy; functional structure.
Conflict of interest statement
CONFLICTS OF INTEREST None.
Figures

References
-
- Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. - PubMed
-
- Nunez-Prado N, Compte M, Harwood S, Alvarez-Mendez A, Lykkemark S, Sanz L, Alvarez-Vallina L. The coming of age of engineered multivalent antibodies. Drug Discov Today. 2015;20:588–594. - PubMed
-
- Rathi C, Meibohm B. Clinical Pharmacology of Bispecific Antibody Constructs. J Clin Pharmacol. 2015;55:S21–S28. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

