Optical control of GPR40 signalling in pancreatic β-cells
- PMID: 29568424
- PMCID: PMC5848828
- DOI: 10.1039/c7sc01475a
Optical control of GPR40 signalling in pancreatic β-cells
Abstract
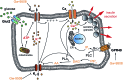
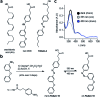
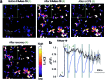
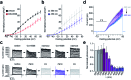
Fatty acids activate GPR40 and K+ channels to modulate β-cell function. Herein, we describe the design and synthesis of FAAzo-10, a light-controllable GPR40 agonist based on Gw-9508. FAAzo-10 is a potent GPR40 agonist in the trans-configuration and can be inactivated on isomerization to cis with UV-A light. Irradiation with blue light reverses this effect, allowing FAAzo-10 activity to be cycled ON and OFF with a high degree of spatiotemporal precision. In dissociated primary mouse β-cells, FAAzo-10 also inactivates voltage-activated and ATP-sensitive K+ channels, and allows us to control glucose-stimulated Ca2+ oscillations in whole islets with light. As such, FAAzo-10 is a useful tool to study the complex effects, with high specificity, which FA-derivatives such as Gw-9508 exert at multiple targets in mouse β-cells.
Figures


Similar articles
-
GPR40 protein levels are crucial to the regulation of stimulated hormone secretion in pancreatic islets. Lessons from spontaneous obesity-prone and non-obese type 2 diabetes in rats.Mol Cell Endocrinol. 2013 Dec 5;381(1-2):150-9. doi: 10.1016/j.mce.2013.07.025. Epub 2013 Aug 2. Mol Cell Endocrinol. 2013. PMID: 23911664
-
Multiple mechanisms of GW-9508, a selective G protein-coupled receptor 40 agonist, in the regulation of glucose homeostasis and insulin sensitivity.Am J Physiol Endocrinol Metab. 2013 Mar 15;304(6):E668-76. doi: 10.1152/ajpendo.00419.2012. Epub 2013 Jan 22. Am J Physiol Endocrinol Metab. 2013. PMID: 23341496
-
Free fatty acids increase cytosolic free calcium and stimulate insulin secretion from beta-cells through activation of GPR40.Mol Cell Endocrinol. 2007 Jan 15;263(1-2):173-80. doi: 10.1016/j.mce.2006.09.013. Epub 2006 Nov 13. Mol Cell Endocrinol. 2007. PMID: 17101212
-
Electrophysiology of islet cells.Adv Exp Med Biol. 2010;654:115-63. doi: 10.1007/978-90-481-3271-3_7. Adv Exp Med Biol. 2010. PMID: 20217497 Review.
-
The Pancreatic β-Cell: The Perfect Redox System.Antioxidants (Basel). 2021 Jan 29;10(2):197. doi: 10.3390/antiox10020197. Antioxidants (Basel). 2021. PMID: 33572903 Free PMC article. Review.
Cited by
-
Optical Approaches for Investigating Neuromodulation and G Protein-Coupled Receptor Signaling.Pharmacol Rev. 2023 Nov;75(6):1119-1139. doi: 10.1124/pharmrev.122.000584. Epub 2023 Jul 10. Pharmacol Rev. 2023. PMID: 37429736 Free PMC article. Review.
-
Mechanistic Insights into Light-Driven Allosteric Control of GPCR Biological Activity.ACS Pharmacol Transl Sci. 2020 Aug 18;3(5):883-895. doi: 10.1021/acsptsci.0c00054. eCollection 2020 Oct 9. ACS Pharmacol Transl Sci. 2020. PMID: 33073188 Free PMC article.
-
Fine-tuned photochromic sulfonylureas for optical control of beta cell Ca2+ fluxes.Diabet Med. 2023 Dec;40(12):e15220. doi: 10.1111/dme.15220. Epub 2023 Sep 21. Diabet Med. 2023. PMID: 37669696 Free PMC article.
-
Photoswitchable Isoprenoid Lipids Enable Optical Control of Peptide Lipidation.ACS Chem Biol. 2022 Oct 21;17(10):2945-2953. doi: 10.1021/acschembio.2c00645. Epub 2022 Oct 4. ACS Chem Biol. 2022. PMID: 36194691 Free PMC article.
-
Selective Photoswitchable Allosteric Agonist of a G Protein-Coupled Receptor.J Am Chem Soc. 2021 Jun 23;143(24):8951-8956. doi: 10.1021/jacs.1c02586. Epub 2021 Jun 11. J Am Chem Soc. 2021. PMID: 34115935 Free PMC article.
References
-
- Salem N., Litman B., Kim H., Gawrisch K. Lipids. 2001;36:945–959. - PubMed
-
- Milligan G., Shimpukade B., Ulven T., Hudson B. D. Chem. Rev. 2017;117(1):67–110. - PubMed
-
- Boland L. M., Drzewiecki M. M. Cell Biochem. Biophys. 2008;52:59–84. - PubMed
-
- IUPAC Gold Book – Fatty Acids, ed. A. D. McNaught and A. Wilkinson, Blackwell Scientific Publications, Oxford, 2nd edn, 1997.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

