Chemoenzymatic synthesis of heparan sulfate and heparin oligosaccharides and NMR analysis: paving the way to a diverse library for glycobiologists
- PMID: 29568440
- PMCID: PMC5849142
- DOI: 10.1039/c7sc03541a
Chemoenzymatic synthesis of heparan sulfate and heparin oligosaccharides and NMR analysis: paving the way to a diverse library for glycobiologists
Abstract
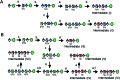
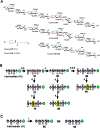
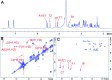
Heparan sulfate (HS) is a member of the glycosaminoglycans (GAG) family that plays essential roles in biological processes from animal sources. Heparin, a highly sulfated form of HS, is widely used as anticoagulant drug worldwide. The high diversity and complexity of HS and heparin represent a roadblock for structural characterization and biological activity studies. Access to structurally defined oligosaccharides is critical for the successful development of HS and heparin structure-activity relationships. In this study, a library of 66 HS and heparin oligosaccharides covering different sulfation patterns and sizes was prepared through an efficient method of chemoenzymatic synthesis. A systematic nuclear magnetic resonance spectroscopy study was firstly undertaken for every oligosaccharide in the library. In addition to the availability of different oligosaccharides, this work also provides spectroscopic data helpful for characterizing more complicated polysaccharide structures providing a safeguard to ensure the quality of the drug heparin. This HS/heparin library will be useful for activity screening and facilitate future structure-activity relationship studies.
Figures


Similar articles
-
Chemoenzymatic Synthesis of Glycosaminoglycans.Acc Chem Res. 2020 Feb 18;53(2):335-346. doi: 10.1021/acs.accounts.9b00420. Epub 2019 Nov 12. Acc Chem Res. 2020. PMID: 31714740
-
Investigation of the biological functions of heparan sulfate using a chemoenzymatic synthetic approach.RSC Chem Biol. 2021 Feb 22;2(3):702-712. doi: 10.1039/d0cb00199f. RSC Chem Biol. 2021. PMID: 34179782 Free PMC article. Review.
-
Chemoenzymatic synthesis of heparan sulfate and heparin.Nat Prod Rep. 2014 Dec;31(12):1676-85. doi: 10.1039/c4np00076e. Epub 2014 Sep 8. Nat Prod Rep. 2014. PMID: 25197032 Free PMC article. Review.
-
Rationally designed chemoenzymatic synthesis of heparan sulfate oligosaccharides with neutralizable anticoagulant activity and low severe complications.Carbohydr Polym. 2025 Jun 1;357:123433. doi: 10.1016/j.carbpol.2025.123433. Epub 2025 Feb 22. Carbohydr Polym. 2025. PMID: 40158971
-
Efficient platform for synthesizing comprehensive heparan sulfate oligosaccharide libraries for decoding glycosaminoglycan-protein interactions.Nat Chem. 2023 Aug;15(8):1108-1117. doi: 10.1038/s41557-023-01248-4. Epub 2023 Jun 22. Nat Chem. 2023. PMID: 37349377 Free PMC article.
Cited by
-
Profiling Heparan Sulfate-Heavy Metal Ions Interaction Using Electrochemical Techniques.Chemistry. 2022 Oct 4;28(55):e202202193. doi: 10.1002/chem.202202193. Epub 2022 Aug 18. Chemistry. 2022. PMID: 35904207 Free PMC article.
-
Multifaceted Heparin: Diverse Applications beyond Anticoagulant Therapy.Pharmaceuticals (Basel). 2024 Oct 12;17(10):1362. doi: 10.3390/ph17101362. Pharmaceuticals (Basel). 2024. PMID: 39459002 Free PMC article. Review.
-
Filter-entrapment enrichment pull-down assay for glycosaminoglycan structural characterization and protein interaction.Carbohydr Polym. 2020 Oct 1;245:116623. doi: 10.1016/j.carbpol.2020.116623. Epub 2020 Jun 10. Carbohydr Polym. 2020. PMID: 32718661 Free PMC article.
-
Controlled release of growth factors using synthetic glycosaminoglycans in a modular macroporous scaffold for tissue regeneration.Commun Biol. 2022 Dec 8;5(1):1349. doi: 10.1038/s42003-022-04305-9. Commun Biol. 2022. PMID: 36482075 Free PMC article.
-
Analysis of 3-O-Sulfated Heparan Sulfate Using Isotopically Labeled Oligosaccharide Calibrants.Anal Chem. 2022 Feb 15;94(6):2950-2957. doi: 10.1021/acs.analchem.1c04965. Epub 2022 Feb 2. Anal Chem. 2022. PMID: 35107975 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

