The α-tertiary amine motif drives remarkable selectivity for Pd-catalyzed carbonylation of β-methylene C-H bonds
- PMID: 29568467
- PMCID: PMC5855970
- DOI: 10.1039/c7sc03876c
The α-tertiary amine motif drives remarkable selectivity for Pd-catalyzed carbonylation of β-methylene C-H bonds
Abstract
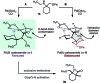
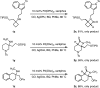
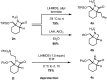
The selective C-H carbonylation of methylene bonds in the presence of traditionally more reactive methyl C-H and C(sp2)-H bonds in α-tertiary amines is reported. The exceptional selectivity is driven by the bulky α-tertiary amine motif, which we hypothesise orientates the activating C-H bond proximal to Pd in order to avoid an unfavourable steric clash with a second α-tertiary amine on the Pd centre, promoting preferential cyclopalladation at the methylene position. The reaction tolerates a range of structurally interesting and synthetically versatile functional groups, delivering the corresponding β-lactam products in good to excellent yields.
Figures



Similar articles
-
Transition Metal-Catalyzed Regioselective Direct C-H Amidation: Interplay between Inner- and Outer-Sphere Pathways for Nitrene Cross-Coupling Reactions.Acc Chem Res. 2022 Aug 2;55(15):2123-2137. doi: 10.1021/acs.accounts.2c00283. Epub 2022 Jul 19. Acc Chem Res. 2022. PMID: 35853135
-
Oxalyl amide assisted palladium-catalyzed synthesis of pyrrolidones via carbonylation of γ-C(sp3)-H bonds of aliphatic amine substrates.Chem Sci. 2015 Aug 1;6(8):4610-4614. doi: 10.1039/c5sc00519a. Epub 2015 May 19. Chem Sci. 2015. PMID: 29619163 Free PMC article.
-
Selective Palladium(II)-Catalyzed Carbonylation of Methylene β-C-H Bonds in Aliphatic Amines.Angew Chem Int Ed Engl. 2017 Sep 18;56(39):11958-11962. doi: 10.1002/anie.201706303. Epub 2017 Aug 21. Angew Chem Int Ed Engl. 2017. PMID: 28707312
-
Transition Metal Catalyzed Free-Amine (-NH2 ) Directed C-H Bond Activation and Functionalization for Biaryl Frameworks.Chem Rec. 2021 Dec;21(12):3795-3817. doi: 10.1002/tcr.202100158. Epub 2021 Jul 8. Chem Rec. 2021. PMID: 34235831 Review.
-
Metal-catalyzed silylation of sp3C-H bonds.Chem Soc Rev. 2021 Apr 26;50(8):5062-5085. doi: 10.1039/d0cs01392g. Chem Soc Rev. 2021. PMID: 33629997 Review.
Cited by
-
Enantioselective Synthesis of N-Alkylamines through β-Amino C-H Functionalization Promoted by Cooperative Actions of B(C6F5)3 and a Chiral Lewis Acid Co-Catalyst.J Am Chem Soc. 2021 Feb 10;143(5):2441-2455. doi: 10.1021/jacs.0c13200. Epub 2021 Jan 29. J Am Chem Soc. 2021. PMID: 33512998 Free PMC article.
-
Second-Generation Palladium Catalyst System for Transannular C-H Functionalization of Azabicycloalkanes.J Am Chem Soc. 2018 Apr 25;140(16):5599-5606. doi: 10.1021/jacs.8b02142. Epub 2018 Apr 13. J Am Chem Soc. 2018. PMID: 29652497 Free PMC article.
-
DP4-AI automated NMR data analysis: straight from spectrometer to structure.Chem Sci. 2020 Mar 6;11(17):4351-4359. doi: 10.1039/d0sc00442a. Chem Sci. 2020. PMID: 34122893 Free PMC article.
-
A comprehensive overview of directing groups applied in metal-catalysed C-H functionalisation chemistry.Chem Soc Rev. 2018 Aug 28;47(17):6603-6743. doi: 10.1039/c8cs00201k. Chem Soc Rev. 2018. PMID: 30033454 Free PMC article. Review.
-
Diastereoselective C-H carbonylative annulation of aliphatic amines: a rapid route to functionalized γ-lactams.Chem Sci. 2018 Jul 31;9(39):7628-7633. doi: 10.1039/c8sc02855a. eCollection 2018 Oct 21. Chem Sci. 2018. PMID: 30393523 Free PMC article.
References
-
-
For examples of C–H activation using auxiliaries see: oximes:
- Desai L. V., Hull K. L., Sanford M. S. J. Am. Chem. Soc. 2004;126:9542. - PubMed
- Giri R., Chen X., Yu J.-Q. Angew. Chem., Int. Ed. 2005;44:2112. - PubMed
- Zaitsev V. G., Shabashov D., Daugulis O. J. Am. Chem. Soc. 2005;127:13154. - PubMed
- Wang D.-H., Wasa M., Giri R., Yu J.-Q. J. Am. Chem. Soc. 2008;130:7190. - PubMed
- Wasa M., Engle K. M., Yu J.-Q. J. Am. Chem. Soc. 2009;131:9886. - PMC - PubMed
- Ren Z., Mo F., Dong G. J. Am. Chem. Soc. 2012;134:16991. - PubMed
- Chan K. S. L., Wasa M., Chu L., Laforteza B. N., Miura M., Yu J.-Q. Nat. Chem. 2014;6:146. - PMC - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources

