The α-tertiary amine motif drives remarkable selectivity for Pd-catalyzed carbonylation of β-methylene C-H bonds
- PMID: 29568467
- PMCID: PMC5855970
- DOI: 10.1039/c7sc03876c
The α-tertiary amine motif drives remarkable selectivity for Pd-catalyzed carbonylation of β-methylene C-H bonds
Abstract
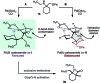
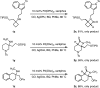
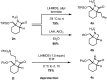
The selective C-H carbonylation of methylene bonds in the presence of traditionally more reactive methyl C-H and C(sp2)-H bonds in α-tertiary amines is reported. The exceptional selectivity is driven by the bulky α-tertiary amine motif, which we hypothesise orientates the activating C-H bond proximal to Pd in order to avoid an unfavourable steric clash with a second α-tertiary amine on the Pd centre, promoting preferential cyclopalladation at the methylene position. The reaction tolerates a range of structurally interesting and synthetically versatile functional groups, delivering the corresponding β-lactam products in good to excellent yields.
Figures



References
-
-
For examples of C–H activation using auxiliaries see: oximes:
- Desai L. V., Hull K. L., Sanford M. S. J. Am. Chem. Soc. 2004;126:9542. - PubMed
- Giri R., Chen X., Yu J.-Q. Angew. Chem., Int. Ed. 2005;44:2112. - PubMed
- Zaitsev V. G., Shabashov D., Daugulis O. J. Am. Chem. Soc. 2005;127:13154. - PubMed
- Wang D.-H., Wasa M., Giri R., Yu J.-Q. J. Am. Chem. Soc. 2008;130:7190. - PubMed
- Wasa M., Engle K. M., Yu J.-Q. J. Am. Chem. Soc. 2009;131:9886. - PMC - PubMed
- Ren Z., Mo F., Dong G. J. Am. Chem. Soc. 2012;134:16991. - PubMed
- Chan K. S. L., Wasa M., Chu L., Laforteza B. N., Miura M., Yu J.-Q. Nat. Chem. 2014;6:146. - PMC - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources

