Hepatotoxicity of Nonsteroidal Anti-Inflammatory Drugs: A Systematic Review of Randomized Controlled Trials
- PMID: 29568654
- PMCID: PMC5820561
- DOI: 10.1155/2018/5253623
Hepatotoxicity of Nonsteroidal Anti-Inflammatory Drugs: A Systematic Review of Randomized Controlled Trials
Abstract
Background: Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most widely used medication in several countries, including Thailand. NSAIDs have been associated with hepatic side effects; however, the frequency of these side effects is uncertain.
Aim of the review: To systematically review published literature on randomized, controlled trials that assessed the risk of clinically significant hepatotoxicity associated with NSAIDs.
Methods: Searches of bibliographic databases EMBASE, PubMed, and the Cochrane Library were conducted up to July 30, 2016, to identify randomized controlled trials of ibuprofen, naproxen, diclofenac, piroxicam, meloxicam, mefenamic acid, indomethacin, celecoxib, and etoricoxib in adults with any disease that provide information on hepatotoxicity outcomes.
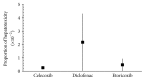
Results: Among the 698 studies, 18 studies met the selection criteria. However, only 8 studies regarding three NSAIDs (celecoxib, etoricoxib, and diclofenac) demonstrated clinically significant hepatotoxic evidence based on hepatotoxicity justification criteria. Of all the hepatotoxicity events found from the above-mentioned three NSAIDs, diclofenac had the highest proportion, which ranged from 0.015 to 4.3 (×10-2), followed by celecoxib, which ranged from 0.13 to 0.38 (×10-2), and etoricoxib, which ranged from 0.005 to 0.930 (×10-2).
Conclusion: Diclofenac had higher rates of hepatotoxic evidence compared to other NSAIDs. Hepatotoxic evidence is mostly demonstrated as aminotransferase elevation, while liver-related hospitalization or discontinuation was very low.
Figures
References
-
- Ornbjerg L. M., Andersen H. B., Kryger P., Cleal B. What do patients in rheumatologic care know about the risks of NSAIDs? J Clin Rheumatol: practical reports on rheumatic & musculoskeletal diseases. 2008;14(2):69–73. - PubMed
-
- Health Product Vigilance Center, Food and Drug Administration, Adverse Events data from 1984, 2016, http://thaihpvc.fda.moph.go.ththaihvcpublic/News/uploads/hpvc_5_13_0_100....
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous