Consistent hypersocial behavior in mice carrying a deletion of Gtf2i but no evidence of hyposocial behavior with Gtf2i duplication: Implications for Williams-Beuren syndrome and autism spectrum disorder
- PMID: 29568691
- PMCID: PMC5853625
- DOI: 10.1002/brb3.895
Consistent hypersocial behavior in mice carrying a deletion of Gtf2i but no evidence of hyposocial behavior with Gtf2i duplication: Implications for Williams-Beuren syndrome and autism spectrum disorder
Abstract
Introduction: Williams-Beuren syndrome (WBS) is a developmental disorder caused by hemizygous deletion of human chromosome 7q11.23. Hypersocial behavior is one symptom of WBS and contrasts with hyposociality observed in autism spectrum disorder (ASD). Interestingly, duplications of 7q11.23 have been associated with ASD. The social phenotype of WBS has been linked to GTF2I or general transcription factor IIi (TFII-I). Duplication of GTF2I has also been associated with ASD.
Methods: We compared mice having either a deletion (Gtf2i+/- ) or duplication (Gtf2i+/dup ) of Gtf2i to wild-type (Gtf2i+/+ ) littermate controls in a series of behavioral tasks including open-field activity monitoring, olfactory probes, a social choice task, social transmission of food preference, habituation-dishabituation, and operant social motivation paradigms.
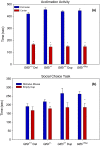
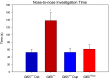
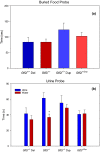
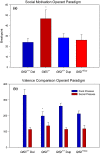
Results: In open-field observations, Gtf2i+/- and Gtf2i+/dup mice demonstrated normal activity and thigmotaxis, and surprisingly, each strain showed a significant preference for a stimulus mouse that was not observed in Gtf2i+/+ siblings. Both Gtf2i+/- and Gtf2i+/dup mice demonstrated normal olfaction in buried food probes, but the Gtf2i+/- mice spent significantly more time investigating urine scent versus water, which was not observed in the other strains. Gtf2i+/- mice also spent significantly more time in nose-to-nose contact compared to Gtf2i+/+ siblings during the open-field encounter of the social transmission of food preference task. In operant tasks of social motivation, Gtf2i+/- mice made significantly more presses for social rewards than Gtf2i+/+ siblings, while there was no difference in presses for the Gtf2i+/dup mice.
Discussion: Results were remarkably consistent across testing paradigms supporting a role for GTF2i in the hypersocial phenotype of WBS and more broadly in the regulation of social behavior. Support was not observed for the role of GTF2i in ASD.
Keywords: 7q11.23; TFII‐I; Williams syndrome; autism; social motivation.
Figures

Similar articles
-
High-throughput screening identifies histone deacetylase inhibitors that modulate GTF2I expression in 7q11.23 microduplication autism spectrum disorder patient-derived cortical neurons.Mol Autism. 2020 Nov 19;11(1):88. doi: 10.1186/s13229-020-00387-6. Mol Autism. 2020. PMID: 33208191 Free PMC article.
-
Molecular investigation, using chromosomal microarray and whole exome sequencing, of six patients affected by Williams Beuren syndrome and Autism Spectrum Disorder.Orphanet J Rare Dis. 2019 May 31;14(1):121. doi: 10.1186/s13023-019-1094-5. Orphanet J Rare Dis. 2019. PMID: 31151468 Free PMC article.
-
Haploinsufficiency of Gtf2i, a gene deleted in Williams Syndrome, leads to increases in social interactions.Autism Res. 2011 Feb;4(1):28-39. doi: 10.1002/aur.169. Epub 2010 Dec 3. Autism Res. 2011. PMID: 21328569
-
The contribution of GTF2I haploinsufficiency to Williams syndrome.Mol Cell Probes. 2018 Aug;40:45-51. doi: 10.1016/j.mcp.2017.12.005. Epub 2018 Jan 3. Mol Cell Probes. 2018. PMID: 29305905 Free PMC article. Review.
-
Williams syndrome deletions and duplications: Genetic windows to understanding anxiety, sociality, autism, and schizophrenia.Neurosci Biobehav Rev. 2017 Aug;79:14-26. doi: 10.1016/j.neubiorev.2017.05.004. Epub 2017 May 10. Neurosci Biobehav Rev. 2017. PMID: 28499504 Review.
Cited by
-
Canine hyper-sociability structural variants associated with altered three-dimensional chromatin state.BMC Genomics. 2024 Aug 7;25(1):767. doi: 10.1186/s12864-024-10614-6. BMC Genomics. 2024. PMID: 39112925 Free PMC article.
-
Deletion of Gtf2i via Systemic Administration of AAV-PHP.eB Virus Increases Social Behavior in a Mouse Model of a Neurodevelopmental Disorder.Biomedicines. 2023 Aug 15;11(8):2273. doi: 10.3390/biomedicines11082273. Biomedicines. 2023. PMID: 37626769 Free PMC article.
-
Functions of Gtf2i and Gtf2ird1 in the developing brain: transcription, DNA binding and long-term behavioral consequences.Hum Mol Genet. 2020 Jun 3;29(9):1498-1519. doi: 10.1093/hmg/ddaa070. Hum Mol Genet. 2020. PMID: 32313931 Free PMC article.
-
GTF2I dosage regulates neuronal differentiation and social behavior in 7q11.23 neurodevelopmental disorders.Sci Adv. 2023 Dec;9(48):eadh2726. doi: 10.1126/sciadv.adh2726. Epub 2023 Nov 29. Sci Adv. 2023. PMID: 38019906 Free PMC article.
-
An IQSEC2 Mutation Associated With Intellectual Disability and Autism Results in Decreased Surface AMPA Receptors.Front Mol Neurosci. 2019 Feb 20;12:43. doi: 10.3389/fnmol.2019.00043. eCollection 2019. Front Mol Neurosci. 2019. PMID: 30842726 Free PMC article.
References
-
- Association , A. P. (2013). Diagnostic and statistical manual of mental disorders. Arlington, VA: American Psychiatric Publishing.
-
- Bariselli, S. , Tzanoulinou, S. , Glangetas, C. , Prevost‐Solie, C. , Pucci, L. , Viguie, J. , … Bellone, C. (2016). SHANK3 controls maturation of social reward circuits in the VTA. Nature Neuroscience, 19, 926–934. https://doi.org/10.1038/nn.4319 - DOI - PMC - PubMed
-
- Bayes, M. , Magano, L. F. , Rivera, N. , Flores, R. , & Perez Jurado, L. A. (2003). Mutational mechanisms of Williams‐Beuren syndrome deletions. American Journal of Human Genetics, 73, 131–151. https://doi.org/10.1086/376565 - DOI - PMC - PubMed
-
- Bellugi, U. , Adolphs, R. , Cassady, C. , & Chiles, M. (1999). Towards the neural basis for hypersociability in a genetic syndrome. NeuroReport, 10, 1653–1657. https://doi.org/10.1097/00001756-199906030-00006 - DOI - PubMed
-
- Bellugi, U. , Korenberg, J. R. , & Klima, E. (2001). Williams Syndrome: An exploration of neurocognitive and genetic features. Clinical Neuroscience Research, 1, 217–229. https://doi.org/10.1016/S1566-2772(01)00008-1 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

