Ratiometric optical nanoprobes enable accurate molecular detection and imaging
- PMID: 29568836
- PMCID: PMC5926823
- DOI: 10.1039/C7CS00612H
Ratiometric optical nanoprobes enable accurate molecular detection and imaging
Abstract
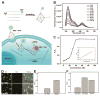
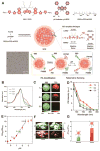
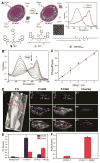
Exploring and understanding biological and pathological changes are of great significance for early diagnosis and therapy of diseases. Optical sensing and imaging approaches have experienced major progress in this field. Particularly, an emergence of various functional optical nanoprobes has provided enhanced sensitivity, specificity, targeting ability, as well as multiplexing and multimodal capabilities due to improvements in their intrinsic physicochemical and optical properties. However, one of the biggest challenges of conventional optical nanoprobes is their absolute intensity-dependent signal readout, which causes inaccurate sensing and imaging results due to the presence of various analyte-independent factors that can cause fluctuations in their absolute signal intensity. Ratiometric measurements provide built-in self-calibration for signal correction, enabling more sensitive and reliable detection. Optimizing nanoprobe designs with ratiometric strategies can surmount many of the limitations encountered by traditional optical nanoprobes. This review first elaborates upon existing optical nanoprobes that exploit ratiometric measurements for improved sensing and imaging, including fluorescence, surface enhanced Raman scattering (SERS), and photoacoustic nanoprobes. Next, a thorough discussion is provided on design strategies for these nanoprobes, and their potential biomedical applications for targeting specific biomolecule populations (e.g. cancer biomarkers and small molecules with physiological relevance), for imaging the tumor microenvironment (e.g. pH, reactive oxygen species, hypoxia, enzyme and metal ions), as well as for intraoperative image guidance of tumor-resection procedures.
Figures










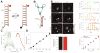








References
-
- Chen M, He X, Wang K, He D, Yang X, Shi H. TrAC, Trends Anal Chem. 2014;58:120–129.
-
- Schulz A, McDonagh C. Soft Matter. 2012;8:2579–2585.
-
- Ueno T, Nagano T. Nat Methods. 2011;8:642–645. - PubMed
-
- Stich MI, Fischer LH, Wolfbeis OS. Chem Soc Rev. 2010;39:3102–3114. - PubMed
-
- Lanza GM, Winter PM, Caruthers SD, Morawski AM, Schmieder AH, Crowder KC, Wickline SA. J Nucl Cardiol. 2004;11:733–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

