Accelerated inflammation and oxidative stress induced by LPS in acute lung injury: Ιnhibition by ST1926
- PMID: 29568857
- PMCID: PMC5881729
- DOI: 10.3892/ijmm.2018.3574
Accelerated inflammation and oxidative stress induced by LPS in acute lung injury: Ιnhibition by ST1926
Abstract
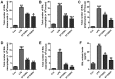
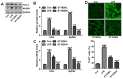
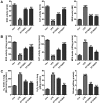
Bioavailable and less toxic synthetic retinoids, such as the atypical adamantyl retinoid ST1926, have been well developed and investigated in clinical trials for many diseases. The aim of our study was to explore the role of ST1926 in lipopolysaccharide (LPS)-induced acute lung injury (ALI) and to reveal the possible molecular mechanism. Mice were treated with LPS to induce acute lung injury followed by ST1926 administration. After LPS induction, mice administered with ST1926 showed lower inflammation infiltration in bronchoalveolar lavage (BAL) fluid, and pro-inflammatory cytokines, including interleukin-1β (IL-1β), IL-18, IL-6 and tumor necrosis factor-α (TNF-α) in serum and lung tissue samples obtained from mice. In addition, western blot assays suggested that ST1926 suppressed nuclear factor-κB (NF-κB), inhibitor-κB kinase-α (IκBα) and IκB kinase (IKKα), as well as Toll-like receptor 4 (TLR4) induced by LPS. In addition, reactive oxygen species (ROS) stimulated by LPS was also suppressed for ST1926 through inhibiting p38 and extracellular receptor kinase (ERK) signaling pathway. Taken together, the data here indicated that ST1926 may be of potential value in treating acute lung injury through inflammation and ROS suppression via inactivating TLR4/NF-κB and p38/ERK1/2 signaling pathways.
Figures




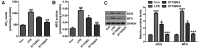






Similar articles
-
Protective effects of polydatin on lipopolysaccharide-induced acute lung injury through TLR4-MyD88-NF-κB pathway.Int Immunopharmacol. 2015 Dec;29(2):370-376. doi: 10.1016/j.intimp.2015.10.027. Epub 2015 Oct 24. Int Immunopharmacol. 2015. PMID: 26507165
-
Kegan Liyan oral liquid ameliorates lipopolysaccharide-induced acute lung injury through inhibition of TLR4-mediated NF-κB signaling pathway and MMP-9 expression.J Ethnopharmacol. 2016 Jun 20;186:91-102. doi: 10.1016/j.jep.2016.03.057. Epub 2016 Mar 30. J Ethnopharmacol. 2016. PMID: 27036629
-
Cavidine Ameliorates Lipopolysaccharide-Induced Acute Lung Injury via NF-κB Signaling Pathway in vivo and in vitro.Inflammation. 2017 Aug;40(4):1111-1122. doi: 10.1007/s10753-017-0553-1. Inflammation. 2017. PMID: 28365871
-
The effect of magnolol on the Toll-like receptor 4/nuclear factor κB signaling pathway in lipopolysaccharide-induced acute lung injury in mice.Eur J Pharmacol. 2012 Aug 15;689(1-3):255-61. doi: 10.1016/j.ejphar.2012.05.038. Epub 2012 Jun 7. Eur J Pharmacol. 2012. PMID: 22683864
-
Kaempferol regulates MAPKs and NF-κB signaling pathways to attenuate LPS-induced acute lung injury in mice.Int Immunopharmacol. 2012 Oct;14(2):209-16. doi: 10.1016/j.intimp.2012.07.007. Epub 2012 Jul 23. Int Immunopharmacol. 2012. PMID: 22835426
Cited by
-
Fmr1 protects cardiomyocytes against lipopolysaccharide-induced myocardial injury.Exp Ther Med. 2018 Sep;16(3):1825-1833. doi: 10.3892/etm.2018.6386. Epub 2018 Jul 2. Exp Ther Med. 2018. PMID: 30186407 Free PMC article.
-
Effect of Apremilast on LPS-induced immunomodulation and inflammation via activation of Nrf2/HO-1 pathways in rat lungs.Saudi Pharm J. 2023 Jul;31(7):1327-1338. doi: 10.1016/j.jsps.2023.05.022. Epub 2023 May 29. Saudi Pharm J. 2023. PMID: 37323920 Free PMC article.
-
Galuminox: Preclinical validation of a novel PET tracer for non-invasive imaging of oxidative stress in vivo.Redox Biol. 2020 Oct;37:101690. doi: 10.1016/j.redox.2020.101690. Epub 2020 Aug 21. Redox Biol. 2020. PMID: 33039825 Free PMC article.
-
Tanshinone IIA loaded bioactive nanoemulsion for alleviation of lipopolysaccharide induced acute lung injury via inhibition of endothelial glycocalyx shedding.Biomed Pharmacother. 2022 Nov;155:113666. doi: 10.1016/j.biopha.2022.113666. Epub 2022 Sep 12. Biomed Pharmacother. 2022. PMID: 36099790 Free PMC article.
-
Plant polysaccharides with anti-lung injury effects as a potential therapeutic strategy for COVID-19.Front Pharmacol. 2022 Oct 20;13:982893. doi: 10.3389/fphar.2022.982893. eCollection 2022. Front Pharmacol. 2022. PMID: 36339607 Free PMC article. Review.
References
-
- Rettig JS, Smallwood CD, Walsh BK, Rimensberger PC, Bachman TE, Bollen CW, Duval EL, Gebistorf F, Markhorst DG, Tinnevelt M, et al. High-frequency oscillatory ventilation in pediatric acute lung injury: A multicenter international experience. Crit Care Med. 2015;43:2660–2667. doi: 10.1097/CCM.0000000000001278. - DOI - PubMed
-
- López-Fernández Y, Azagra AM, de la Oliva P, Modesto V, Sánchez JI, Parrilla J, Arroyo MJ, Reyes SB, Pons-Ódena M, López-Herce J, et al. Pediatric Acute Lung Injury Epidemiology and Natural History (PED-ALIEN) Network: Pediatric acute lung injury epidemiology and natural history study: Incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med. 2012;40:3238–3245. doi: 10.1097/CCM.0b013e318260caa3. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

