Fisetin administration improves LPS-induced acute otitis media in mouse in vivo
- PMID: 29568876
- PMCID: PMC5979934
- DOI: 10.3892/ijmm.2018.3585
Fisetin administration improves LPS-induced acute otitis media in mouse in vivo
Abstract
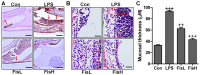
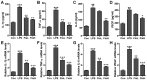
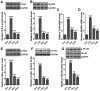
Acute otitis media is one of the most common infectious diseases worldwide in spite of the widespread vaccination. The present study was conducted to explore the effects of fisetin on mouse acute otitis media models. The animal models were established by lipopolysaccharide (LPS) injection into the middle ear of mice via the tympanic membrane. Fisetin was administered to mice for ten days through intragastric administration immediate after LPS application. Hematoxylin and eosin (H&E) staining was performed and the pro-inflammatory cytokines, including interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), IL-6 and VEGF, were measured through enzyme-linked immunosorbent assay (ELISA) method and RT-qPCR analysis. Toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) signaling pathway was detected by immunoblotting assays. Reactive oxygen species (ROS) generated levels were determined through assessment of anti-oxidants, and TXNIP/MAPKs signaling pathways were explored to reveal the possible molecular mechanism for acute otitis media progression and the function of fisetin. Fisetin reduced mucosal thickness caused by LPS. In fisetin-treated animals, pro-inflammatory cytokine release was downregulated accompanied with TLR4/NF-κB inactivation. ROS production was significantly decreased in comparison to the LPS-treated group. The TXNIP/MAPKs signaling pathway was inactivated for fisetin treatment in LPS-induced mice with acute otitis media. The above results indicated that fisetin improved acute otitis media through inflammation and ROS suppression via inactivating TLR4/NF-κB and TXNIP/MAPKs signaling pathways.
Figures





Similar articles
-
Apigetrin treatment attenuates LPS-induced acute otitis media though suppressing inflammation and oxidative stress.Biomed Pharmacother. 2019 Jan;109:1978-1987. doi: 10.1016/j.biopha.2018.07.022. Epub 2018 Nov 26. Biomed Pharmacother. 2019. PMID: 30551453
-
Digitoflavone (DG) attenuates LPS-induced acute lung injury through reducing oxidative stress and inflammatory response dependent on the suppression of TXNIP/NLRP3 and NF-κB.Biomed Pharmacother. 2017 Oct;94:712-725. doi: 10.1016/j.biopha.2017.07.001. Epub 2017 Aug 8. Biomed Pharmacother. 2017. PMID: 28800542
-
Fisetin Alleviates Lipopolysaccharide-Induced Acute Lung Injury via TLR4-Mediated NF-κB Signaling Pathway in Rats.Inflammation. 2016 Feb;39(1):148-157. doi: 10.1007/s10753-015-0233-y. Inflammation. 2016. PMID: 26272311
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
The Potential Role of Fisetin, a Flavonoid in Cancer Prevention and Treatment.Molecules. 2022 Dec 17;27(24):9009. doi: 10.3390/molecules27249009. Molecules. 2022. PMID: 36558146 Free PMC article. Review.
Cited by
-
Role of Thioredoxin-Interacting Protein in Diseases and Its Therapeutic Outlook.Int J Mol Sci. 2021 Mar 9;22(5):2754. doi: 10.3390/ijms22052754. Int J Mol Sci. 2021. PMID: 33803178 Free PMC article. Review.
-
TXNIP: A key protein in the cellular stress response pathway and a potential therapeutic target.Exp Mol Med. 2023 Jul;55(7):1348-1356. doi: 10.1038/s12276-023-01019-8. Epub 2023 Jul 3. Exp Mol Med. 2023. PMID: 37394581 Free PMC article. Review.
-
Silencing Nrf2 attenuates chronic suppurative otitis media by inhibiting pro-inflammatory cytokine secretion through up-regulating TLR4.Innate Immun. 2021 Jan;27(1):70-80. doi: 10.1177/1753425920933661. Epub 2020 Jun 24. Innate Immun. 2021. PMID: 32579053 Free PMC article.
-
BA inhibits LPS-stimulated inflammatory response and apoptosis in human middle ear epithelial cells by regulating the Nf-Kb/Iκbα axis.Open Life Sci. 2024 Dec 31;19(1):20221019. doi: 10.1515/biol-2022-1019. eCollection 2024. Open Life Sci. 2024. PMID: 39822380 Free PMC article.
-
Fisetin inhibits inflammation and induces autophagy by mediating PI3K/AKT/mTOR signaling in LPS-induced RAW264.7 cells.Food Nutr Res. 2021 Mar 25;65. doi: 10.29219/fnr.v65.6355. eCollection 2021. Food Nutr Res. 2021. PMID: 33841067 Free PMC article.
References
-
- Bluestone CD, Klein JO. Physiology, pathophysiology and pathogenesis. Decker BC, editor. Otitis Media in Infants and Children. (4th edition) 2007:41–42.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

