Quercetin inhibits NTHi-triggered CXCR4 activation through suppressing IKKα/NF-κB and MAPK signaling pathways in otitis media
- PMID: 29568908
- PMCID: PMC5979834
- DOI: 10.3892/ijmm.2018.3577
Quercetin inhibits NTHi-triggered CXCR4 activation through suppressing IKKα/NF-κB and MAPK signaling pathways in otitis media
Abstract
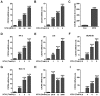
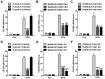
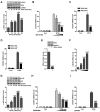
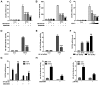
Otitis media is one of the most common bacterial infections in children, contributing to hearing loss. A vital bacterial pathogen leading to otitis media development is the nontypeable Haemophilus influenzae (NTHi). Inflammation response is reported as an important characristic for otitis media. Chemokine CXC receptor 4 (CXCR4) is a 352-amino acid seven-span transmembrane G-protein coupled receptor, essential for inflammatory response. However, the possible molecular mechanism indicating the alteration of CXCR4 modulated by NTHi is poorly known. In the present study, NTHi enhanced CXCR4 expression through phosphorylation of IKKα and p38, which relied on nuclear factor-κB (NF-κB) translocation in vitro as well as in the middle ear of mice in vivo. Previously, quercetin, a natural production mainly isolated from rutin, has shown anti-inflammatory effects. Here, we report that quercetin suppressed NTHi-induced CXCR4 expression levels in vitro and in vivo. Quercetin blocked CXCR4 activation through direct IKKβ phosphorylation inhibition, as well as of p38 MAPK restraining. Hence, identification of quercetin may be a potential therapeutic strategy for treating otitis media induced by NTHi through inflammation suppression.
Figures

Similar articles
-
Activation of NF-kappa B by nontypeable Hemophilus influenzae is mediated by toll-like receptor 2-TAK1-dependent NIK-IKK alpha /beta-I kappa B alpha and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells.Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8774-9. doi: 10.1073/pnas.151236098. Epub 2001 Jul 3. Proc Natl Acad Sci U S A. 2001. PMID: 11438700 Free PMC article.
-
Nontypeable Haemophilus influenzae induces COX-2 and PGE2 expression in lung epithelial cells via activation of p38 MAPK and NF-kappa B.Respir Res. 2008 Jan 31;9(1):16. doi: 10.1186/1465-9921-9-16. Respir Res. 2008. PMID: 18237405 Free PMC article.
-
Synergistic activation of NF-kappaB by nontypeable Haemophilus influenzae and tumor necrosis factor alpha.Proc Natl Acad Sci U S A. 2004 Mar 9;101(10):3563-8. doi: 10.1073/pnas.0400557101. Epub 2004 Mar 1. Proc Natl Acad Sci U S A. 2004. PMID: 14993593 Free PMC article.
-
Exploitation of host epithelial signaling networks by respiratory bacterial pathogens.J Pharmacol Sci. 2003 Jan;91(1):1-7. doi: 10.1254/jphs.91.1. J Pharmacol Sci. 2003. PMID: 12686724 Review.
-
A Comprehensive View on the Quercetin Impact on Colorectal Cancer.Molecules. 2022 Mar 14;27(6):1873. doi: 10.3390/molecules27061873. Molecules. 2022. PMID: 35335239 Free PMC article. Review.
Cited by
-
Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds.Oxid Med Cell Longev. 2022 Sep 6;2022:9966750. doi: 10.1155/2022/9966750. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36111166 Free PMC article. Review.
-
BA inhibits LPS-stimulated inflammatory response and apoptosis in human middle ear epithelial cells by regulating the Nf-Kb/Iκbα axis.Open Life Sci. 2024 Dec 31;19(1):20221019. doi: 10.1515/biol-2022-1019. eCollection 2024. Open Life Sci. 2024. PMID: 39822380 Free PMC article.
-
Identification of potential targets of triptolide in regulating the tumor microenvironment of stomach adenocarcinoma patients using bioinformatics.Bioengineered. 2021 Dec;12(1):4304-4319. doi: 10.1080/21655979.2021.1945522. Bioengineered. 2021. PMID: 34348580 Free PMC article.
-
Interactions of selected cardiovascular active natural compounds with CXCR4 and CXCR7 receptors: a molecular docking, molecular dynamics, and pharmacokinetic/toxicity prediction study.BMC Complement Med Ther. 2022 Feb 4;22(1):35. doi: 10.1186/s12906-021-03488-8. BMC Complement Med Ther. 2022. PMID: 35120520 Free PMC article.
-
miR-142-5p as a CXCR4-Targeted MicroRNA Attenuates SDF-1-Induced Chondrocyte Apoptosis and Cartilage Degradation via Inactivating MAPK Signaling Pathway.Biochem Res Int. 2020 Jan 24;2020:4508108. doi: 10.1155/2020/4508108. eCollection 2020. Biochem Res Int. 2020. PMID: 32047668 Free PMC article.
References
-
- Bluestone CD, Klein JO. Physiology, pathophysiology and pathogenesis Otitis Media in Infants and Children. 4th edition. BC Decker; 2007. pp. 41–42.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

