A novel therapeutic strategy of personalized medicine based on anti-epidermal growth factor receptor monoclonal antibodies in patients with metastatic colorectal cancer
- PMID: 29568913
- PMCID: PMC5873832
- DOI: 10.3892/ijo.2018.4322
A novel therapeutic strategy of personalized medicine based on anti-epidermal growth factor receptor monoclonal antibodies in patients with metastatic colorectal cancer
Abstract
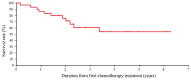
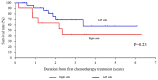
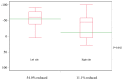
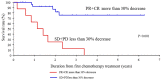
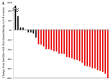
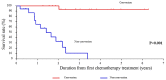
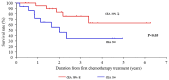
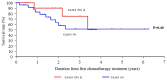
Achieving tumor shrinkage may be a clinically relevant improvement in the treatment of metastatic colorectal cancer (mCRC). The present study attempted to evaluate early tumor shrinkage (ETS) and deepness of response over 6-8 courses of therapy, which were assessed previously in first-line trials of anti-epidermal growth factor receptor (EGFR) monoclonal antibodies. A total of 37 patients with mCRC that was considered unresectable or borderline resectable were enrolled in the study. Patients exhibited the wild-type RAS gene, and anti-EGFR monoclonal antibodies were used as the first-line treatment in the Department of Surgical Oncology at Gifu University School of Medicine (Gifu, Japan) between January 2010 and March 2017. Tumor shrinkage and other characteristics were evaluated according to the Response Evaluation Criteria In Solid Tumors (RECIST) classifications (version 1.1). The 3-year overall survival (OS) rate was >60.0% for all cases (n=37). The mean tumor shrinkage rate in the right side of the colon according to the RECIST classifications was -11.1%, whereas that for CRC on the left side showed a statistically significant difference at -54.0% (P=0.042). In addition, the rates of OS for stable disease + progressive disease compared with partial response + complete response, and those of OS for conversion therapy compared with non-conversion therapy were significantly different (both P<0.001). Carcinoembryonic antigen (CEA) was suggested to be a possible predictive factor for convalescence due to the 50% drop in its value after the 6-8 courses of therapy. Overall, the predictive performance of ETS with respect to PFS and OS is at least as good as the standard RECIST response, with the advantage of an earlier assessment, and this may improve convalescence, with CEA as a marker in support of ETS over a clinical treatment course. In RAS wild-type patients, it is important to evaluate the rate of tumor shrinkage from the beginning of the first-line treatment until 6-8 courses of anti-EGFR monoclonal antibodies have been administered.
Conflict of interest statement
KYo has received honoraria for lectures from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Eli Lilly and Company, Daiichi Sankyo Co., Ltd., Ono Pharmaceutical Co., Ltd., Merck Serono Co., Ltd., Novartis Pharma K.K. and Sanofi K.K., and research funding from Ajinomoto Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Taiho Pharmaceutical Co., Ono Pharmaceutical Co., and Yakult Honsha Co., Ltd., outside the submitted work. TaT has received honoraria for lectures from Takeda Pharmaceutical Co., Ltd. All remaining authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Final Analysis of Outcomes and RAS/BRAF Status in a Randomized Phase 3 Study of Panitumumab and Best Supportive Care in Chemorefractory Wild Type KRAS Metastatic Colorectal Cancer.Clin Colorectal Cancer. 2018 Sep;17(3):206-214. doi: 10.1016/j.clcc.2018.03.008. Epub 2018 Mar 21. Clin Colorectal Cancer. 2018. PMID: 29703606 Clinical Trial.
-
TIMP-1 and CEA as biomarkers in third-line treatment with irinotecan and cetuximab for metastatic colorectal cancer.Tumour Biol. 2015 Jun;36(6):4301-8. doi: 10.1007/s13277-015-3069-z. Epub 2015 Jan 23. Tumour Biol. 2015. PMID: 25608838
-
A study-level meta-analysis of efficacy data from head-to-head first-line trials of epidermal growth factor receptor inhibitors versus bevacizumab in patients with RAS wild-type metastatic colorectal cancer.Eur J Cancer. 2016 Nov;67:11-20. doi: 10.1016/j.ejca.2016.07.019. Epub 2016 Sep 1. Eur J Cancer. 2016. PMID: 27592068
-
Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review.Clin Ther. 2010 Mar;32(3):437-53. doi: 10.1016/j.clinthera.2010.03.012. Clin Ther. 2010. PMID: 20399983 Review.
-
Anti-epidermal growth factor receptor monoclonal antibodies in metastatic colorectal cancer: a meta-analysis.World J Gastroenterol. 2015 Apr 14;21(14):4365-72. doi: 10.3748/wjg.v21.i14.4365. World J Gastroenterol. 2015. PMID: 25892888 Free PMC article. Review.
Cited by
-
Cytochrome P450 2U1 Is a Novel Independent Prognostic Biomarker in Breast Cancer Patients.Front Oncol. 2020 Aug 5;10:1379. doi: 10.3389/fonc.2020.01379. eCollection 2020. Front Oncol. 2020. PMID: 32850442 Free PMC article.
-
EYA2 Correlates With Clinico-Pathological Features of Breast Cancer, Promotes Tumor Proliferation, and Predicts Poor Survival.Front Oncol. 2019 Jan 29;9:26. doi: 10.3389/fonc.2019.00026. eCollection 2019. Front Oncol. 2019. PMID: 30761270 Free PMC article.
-
Synchronous liver metastases and peritoneal carcinomatosis from colorectal cancer: different strategies for curative treatment?Langenbecks Arch Surg. 2019 Jun;404(4):477-488. doi: 10.1007/s00423-019-01787-w. Epub 2019 Apr 25. Langenbecks Arch Surg. 2019. PMID: 31025165
-
Pharmacogenetics as a Future Tool to Risk-Stratify Breast Cancer Patients According to Chemotoxicity Potential from the Doxorubicin Hydrochloride and Cyclophosphamide (AC) Regimen.Pharmaceuticals (Basel). 2025 Apr 7;18(4):539. doi: 10.3390/ph18040539. Pharmaceuticals (Basel). 2025. PMID: 40283974 Free PMC article.
-
Ion Channel Targeting with Antibodies and Antibody Fragments for Cancer Diagnosis.Antibodies (Basel). 2019 May 24;8(2):33. doi: 10.3390/antib8020033. Antibodies (Basel). 2019. PMID: 31544839 Free PMC article. Review.
References
-
- Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–450. doi: 10.1097/01.sla.0000138076.72547.b1. - DOI - PMC - PubMed
-
- Arkenau HT, Arnold D, Cassidy J, Diaz-Rubio E, Douillard JY, Hochster H, Martoni A, Grothey A, Hinke A, Schmiegel W, et al. Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: A pooled analysis of randomized trials. J Clin Oncol. 2008;26:5910–5917. doi: 10.1200/JCO.2008.16.7759. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

