A novel therapeutic strategy of personalized medicine based on anti-epidermal growth factor receptor monoclonal antibodies in patients with metastatic colorectal cancer
- PMID: 29568913
- PMCID: PMC5873832
- DOI: 10.3892/ijo.2018.4322
A novel therapeutic strategy of personalized medicine based on anti-epidermal growth factor receptor monoclonal antibodies in patients with metastatic colorectal cancer
Abstract
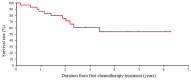
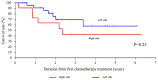
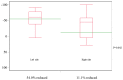
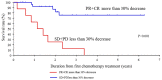
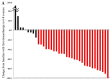
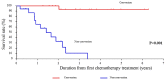
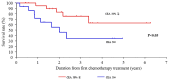
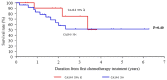
Achieving tumor shrinkage may be a clinically relevant improvement in the treatment of metastatic colorectal cancer (mCRC). The present study attempted to evaluate early tumor shrinkage (ETS) and deepness of response over 6-8 courses of therapy, which were assessed previously in first-line trials of anti-epidermal growth factor receptor (EGFR) monoclonal antibodies. A total of 37 patients with mCRC that was considered unresectable or borderline resectable were enrolled in the study. Patients exhibited the wild-type RAS gene, and anti-EGFR monoclonal antibodies were used as the first-line treatment in the Department of Surgical Oncology at Gifu University School of Medicine (Gifu, Japan) between January 2010 and March 2017. Tumor shrinkage and other characteristics were evaluated according to the Response Evaluation Criteria In Solid Tumors (RECIST) classifications (version 1.1). The 3-year overall survival (OS) rate was >60.0% for all cases (n=37). The mean tumor shrinkage rate in the right side of the colon according to the RECIST classifications was -11.1%, whereas that for CRC on the left side showed a statistically significant difference at -54.0% (P=0.042). In addition, the rates of OS for stable disease + progressive disease compared with partial response + complete response, and those of OS for conversion therapy compared with non-conversion therapy were significantly different (both P<0.001). Carcinoembryonic antigen (CEA) was suggested to be a possible predictive factor for convalescence due to the 50% drop in its value after the 6-8 courses of therapy. Overall, the predictive performance of ETS with respect to PFS and OS is at least as good as the standard RECIST response, with the advantage of an earlier assessment, and this may improve convalescence, with CEA as a marker in support of ETS over a clinical treatment course. In RAS wild-type patients, it is important to evaluate the rate of tumor shrinkage from the beginning of the first-line treatment until 6-8 courses of anti-EGFR monoclonal antibodies have been administered.
Conflict of interest statement
KYo has received honoraria for lectures from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Eli Lilly and Company, Daiichi Sankyo Co., Ltd., Ono Pharmaceutical Co., Ltd., Merck Serono Co., Ltd., Novartis Pharma K.K. and Sanofi K.K., and research funding from Ajinomoto Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Taiho Pharmaceutical Co., Ono Pharmaceutical Co., and Yakult Honsha Co., Ltd., outside the submitted work. TaT has received honoraria for lectures from Takeda Pharmaceutical Co., Ltd. All remaining authors declare that they have no conflicts of interest.
Figures


References
-
- Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–450. doi: 10.1097/01.sla.0000138076.72547.b1. - DOI - PMC - PubMed
-
- Arkenau HT, Arnold D, Cassidy J, Diaz-Rubio E, Douillard JY, Hochster H, Martoni A, Grothey A, Hinke A, Schmiegel W, et al. Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: A pooled analysis of randomized trials. J Clin Oncol. 2008;26:5910–5917. doi: 10.1200/JCO.2008.16.7759. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

