FSCN‑1 increases doxorubicin resistance in hepatocellular carcinoma through promotion of epithelial-mesenchymal transition
- PMID: 29568938
- PMCID: PMC5873898
- DOI: 10.3892/ijo.2018.4327
FSCN‑1 increases doxorubicin resistance in hepatocellular carcinoma through promotion of epithelial-mesenchymal transition
Abstract
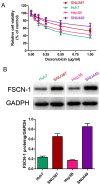
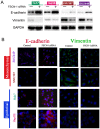
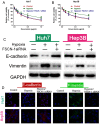
Resistance to chemotherapy drugs remains a significant problem for the treatment of many types of cancer. Fascin‑1 (FSCN‑1) is an actin-bundling protein involved in the invasion and metastasis of a variety of tumors. However, its involvement in drug resistance in hepatocellular carcinoma (HCC) remains unclear. The present study aimed to investigate the function of FSCN‑1 in HCC resistance to doxorubicin (DOX). FSCN‑1 expression was increased in DOX-resistant HCC cell lines (SNU449 and SNU387) compared with DOX-sensitive cell lines (Huh7 and Hep3B). The resistance of HCC cells to DOX was decreased following FSCN‑1 knockdown with small interfering RNA. FSCN‑1 knockdown also significantly altered the expression of key markers of epithelial-mesenchymal transition (EMT). Notably, vimentin expression was reduced and epithelial‑cadherin expression was increased. Furthermore, when EMT was suppressed through knockdown of Twist, an essential pathway of DOX-induced EMT, the viability of HCC cells following treatment with DOX was not affected by FSCN‑1 expression. Furthermore, FSCN‑1 knockdown eliminated hypoxia-induced doxorubicin resistance and EMT. The results of the present study indicated that FSCN‑1 expression increased DOX resistance in HCC cells via the promotion of EMT, and this phenomenon was maintained in a hypoxic environment. FSCN‑1 potentially represents a novel target to overcome resistance to DOX in HCC.
Figures



References
-
- Piska K, Koczurkiewicz P, Bucki A, Wójcik-Pszczoła K, Kołaczkowski M, Pękala E. Metabolic carbonyl reduction of anthracyclines - role in cardiotoxicity and cancer resistance. Reducing enzymes as putative targets for novel cardioprotective and chemosensitizing agents. Invest New Drugs. 2017;35:375–385. doi: 10.1007/s10637-017-0443-2. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

