Identification of clinically relevant phenotypes in patients with Ebstein anomaly
- PMID: 29569399
- PMCID: PMC6489938
- DOI: 10.1002/clc.22870
Identification of clinically relevant phenotypes in patients with Ebstein anomaly
Abstract
Background: Ebstein anomaly (EA) is a heterogeneous congenital heart defect (CHD), frequently accompanied by diverse cardiac and extracardiac comorbidities, resulting in a wide range of clinical outcomes.
Hypothesis: Phenotypic characterization of EA patients has the potential to identify variables that influence prognosis and subgroups with distinct contributing factors.
Methods: A comprehensive cross-sectional phenotypic characterization of 147 EA patients from one of the main referral institutions for CHD in Colombia was carried out. The most prevalent comorbidities and distinct subgroups within the patient cohort were identified through cluster analysis.
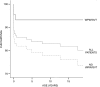
Results: The most prevalent cardiac comorbidities identified were atrial septal defect (61%), Wolff-Parkinson-White syndrome (WPW; 27%), and right ventricular outflow tract obstruction (25%). Cluster analysis showed that patients can be classified into 2 distinct subgroups with defined phenotypes that determine disease severity and survival. Patients in cluster 1 represented a particularly homogeneous subgroup with a milder spectrum of disease, including only patients with WPW and/or supraventricular tachycardia (SVT). Cluster 2 included patients with more diverse cardiovascular comorbidities.
Conclusions: This study represents one of the largest phenotypic characterizations of EA patients reported. The data show that EA is a heterogeneous disease, very frequently associated with cardiovascular and noncardiovascular comorbidities. Patients with WPW and SVT represent a homogeneous subgroup that presents with a less severe spectrum of disease and better survival when adequately managed. This should be considered when searching for genetic causes of EA and in the clinical setting.
Keywords: Congenital Heart Defect; Ebstein Anomaly; Epidemiology; Genetics; Phenotype.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures

References
-
- Kouchoukos NT, Kirklin JW. Ebstein anomaly In: Kouchoukos NT, Blackstone EH, Hanley FL, et al, eds. Cardiac Surgery: Morphology, Diagnostic Criteria, Natural History, Techniques, Results, and Indications. 4th ed Philadelphia, PA: Elsevier/Saunders; 2013:1576–1598.
-
- Andelfinger GU. Human genetics of Ebstein anomaly. In: Rickert‐Sperling S, Kelly RG, Driscoll DJ, eds. Congenital Heart Diseases: The Broken Heart—Clinical Features, Human Genetics and Molecular Pathways. Vienna, Austria: Springer; 2016:613–620.
-
- Lupo PJ, Langlois PH, Mitchell LE. Epidemiology of Ebstein anomaly: prevalence and patterns in Texas, 1999–2005. Am J Med Genet A. 2011;155A:1007–1014. - PubMed
-
- Vermeer AM, van Engelen K, Postma AV, et al. Ebstein anomaly associated with left ventricular noncompaction: an autosomal dominant condition that can be caused by mutations in MYH7. Am J Med Genet C Semin Med Genet. 2013;163C:178–184. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

