Cytochrome P450 3A Induction Predicts P-glycoprotein Induction; Part 2: Prediction of Decreased Substrate Exposure After Rifabutin or Carbamazepine
- PMID: 29569712
- PMCID: PMC6282692
- DOI: 10.1002/cpt.1072
Cytochrome P450 3A Induction Predicts P-glycoprotein Induction; Part 2: Prediction of Decreased Substrate Exposure After Rifabutin or Carbamazepine
Abstract
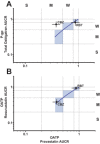
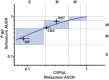
Rifampin demonstrated dose-dependent relative induction between cytochrome P (CYP)3A and P-glycoprotein (P-gp), organic anion transporting polypeptides (OATPs), or CYP2C9; P-gp, OATP, and CYP2C9 induction was one drug-drug interaction (DDI) category lower than that observed for CYP3A across a wide range of pregnane X receptor (PXR) agonism. The objective of this study was to determine if these relationships could be utilized to predict transporter induction by other CYP3A inducers (rifabutin and carbamazepine) and of another P-gp substrate, sofosbuvir. Healthy subjects received sofosbuvir and a six-probe drug cassette before and after 300 mg q.d. rifabutin or 300 mg b.i.d. carbamazepine. Induction of P-gp, CYP2C9, and decreased sofosbuvir exposure were successfully predicted by observed CYP3A induction. Carbamazepine induction of OATP was underpredicted, likely due to reported additional non-PXR agonism. The results demonstrate that the effect of a PXR agonist on CYP3A can be leveraged to inform on induction liability for other primarily PXR-regulated P450s/transporters, allowing for prioritization of targeted DDI assessments during new drug development.
© 2018 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures


Similar articles
-
Cytochrome P450 3A Induction Predicts P-glycoprotein Induction; Part 1: Establishing Induction Relationships Using Ascending Dose Rifampin.Clin Pharmacol Ther. 2018 Dec;104(6):1182-1190. doi: 10.1002/cpt.1073. Epub 2018 Apr 19. Clin Pharmacol Ther. 2018. PMID: 29569723 Free PMC article. Clinical Trial.
-
Acute and Chronic Effects of Rifampin on Letermovir Suggest Transporter Inhibition and Induction Contribute to Letermovir Pharmacokinetics.Clin Pharmacol Ther. 2022 Mar;111(3):664-675. doi: 10.1002/cpt.2510. Epub 2022 Jan 15. Clin Pharmacol Ther. 2022. PMID: 34888851
-
Effects of carbamazepine on the P-gp and CYP3A expression correlated with PXR or NF-κB activity in the bEnd.3 cells.Neurosci Lett. 2019 Jan 18;690:48-55. doi: 10.1016/j.neulet.2018.10.016. Epub 2018 Oct 9. Neurosci Lett. 2019. PMID: 30312753
-
Effect of P-glycoprotein (P-gp) Inducers on Exposure of P-gp Substrates: Review of Clinical Drug-Drug Interaction Studies.Clin Pharmacokinet. 2020 Jun;59(6):699-714. doi: 10.1007/s40262-020-00867-1. Clin Pharmacokinet. 2020. PMID: 32052379 Free PMC article. Review.
-
Drugs as CYP3A probes, inducers, and inhibitors.Drug Metab Rev. 2007;39(4):699-721. doi: 10.1080/03602530701690374. Drug Metab Rev. 2007. PMID: 18058330 Review.
Cited by
-
Cytochrome P450 3A Induction Predicts P-glycoprotein Induction; Part 1: Establishing Induction Relationships Using Ascending Dose Rifampin.Clin Pharmacol Ther. 2018 Dec;104(6):1182-1190. doi: 10.1002/cpt.1073. Epub 2018 Apr 19. Clin Pharmacol Ther. 2018. PMID: 29569723 Free PMC article. Clinical Trial.
-
Drug-Drug Interactions of Direct Oral Anticoagulants (DOACs): From Pharmacological to Clinical Practice.Pharmaceutics. 2022 May 24;14(6):1120. doi: 10.3390/pharmaceutics14061120. Pharmaceutics. 2022. PMID: 35745692 Free PMC article. Review.
-
Tenofovir alafenamide and rifabutin co-administration does not lead to loss of HIV-1 suppression: A retrospective observational study.Int J Infect Dis. 2020 Nov;100:470-472. doi: 10.1016/j.ijid.2020.09.1423. Epub 2020 Sep 24. Int J Infect Dis. 2020. PMID: 32979587 Free PMC article.
-
Drug-drug interaction between rifabutin and dolutegravir: A population pharmacokinetic model.Br J Clin Pharmacol. 2023 Mar;89(3):1216-1221. doi: 10.1111/bcp.15604. Epub 2022 Dec 1. Br J Clin Pharmacol. 2023. PMID: 36385424 Free PMC article.
-
A practical assessment protocol for clinically relevant P-glycoprotein-mediated drug-drug interactions.Front Pharmacol. 2024 Sep 30;15:1412692. doi: 10.3389/fphar.2024.1412692. eCollection 2024. Front Pharmacol. 2024. PMID: 39403136 Free PMC article.
References
-
- Christians, U. , Schmitz, V. , Haschke, M. Functional interactions between P‐glycoprotein and CYP3A in drug metabolism. Expert Opin. Drug Metab. Toxicol. 1, 641–654 (2005). - PubMed
-
- Francis, G.A. , Fayard, E. , Picard, F. , Auwerx, J. Nuclear receptors and the control of metabolism. Annu. Rev. Physiol. 65, 261–311 (2003). - PubMed
-
- Gerbal‐Chaloin, S. et al Induction of CYP2C genes in human hepatocytes in primary culture. Drug Metab. Dispos. 29, 242–251 (2001). - PubMed
-
- Lemmen, J. , Tozakidis, I.E. & Galla, H.J. Pregnane X receptor upregulates ABC‐transporter Abcg2 and Abcb1 at the blood‐brain barrier. Brain Res. 1491, 1–13 (2013). - PubMed
-
- Synold, T.W. , Dussault, L. & Forman, B.M. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat. Med. 7, 584–590 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

