Regulatory T-cells within bone marrow-derived stem cells actively confer immunomodulatory and neuroprotective effects against stroke
- PMID: 29569981
- PMCID: PMC6727132
- DOI: 10.1177/0271678X18766172
Regulatory T-cells within bone marrow-derived stem cells actively confer immunomodulatory and neuroprotective effects against stroke
Abstract
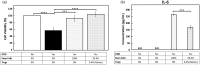
Regulatory T-cells (Tregs) may exert a neuroprotective effect on ischemic stroke by inhibiting both inflammation and effector T-cell activation. Transplantation of human bone marrow-derived stem cells (BMSCs) in ischemic stroke affords neuroprotection that results in part from the cells' anti-inflammatory property. However, the relationship between Tregs and BMSCs in treatment of ischemic stroke has not been fully elucidated. Here, we tested the hypothesis that Tregs within the BMSCs represent active mediators of immunomodulation and neuroprotection in experimental stroke. Primary rat neuronal cells were subjected to an oxygen-glucose deprivation and reperfusion (OGD/R) condition. The cells were re-perfused and co-cultured with Tregs and/or BMSCs. We detected a minority population of Tregs within BMSCs with both immunocytochemistry (ICC) and flow cytometry identifying cells expressing phenotypic markers of CD4, CD25, and FoxP3 protein. BMSCs with the native population of Tregs conferred maximal neuroprotection compared to the treatment conditions containing 0%, 10%, and 100% relative ratio Tregs. Increasing the Treg population resulted in increased IL6 secretion and decreased FGF-β secretion by BMSCs. This study shows that a minority population of Tregs exists within the therapeutic BMSC population, which serves as robust mediators of the immunomodulatory and neuroprotective effect provided by BMSC transplantation.
Keywords: Adaptive immunity; foxp3; immunotherapy; ischemia.
Figures



References
-
- Feigin VL, Lawes CM, Bennett DA, et al. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2003; 2: 43–53. - PubMed
-
- Stevens SL, Bao J, Hollis J, et al. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res 2002; 932: 110–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

