Deletion of choline acetyltransferase in enteric neurons results in postnatal intestinal dysmotility and dysbiosis
- PMID: 29570391
- PMCID: PMC6103169
- DOI: 10.1096/fj.201701474RR
Deletion of choline acetyltransferase in enteric neurons results in postnatal intestinal dysmotility and dysbiosis
Abstract
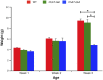
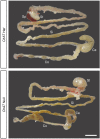
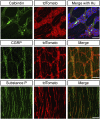
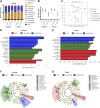
Acetylcholine (ACh)-synthesizing neurons are major components of the enteric nervous system (ENS). They release ACh and peptidergic neurotransmitters onto enteric neurons and muscle. However, pharmacological interrogation has proven inadequate to demonstrate an essential role for ACh. Our objective was to determine whether elimination of ACh synthesis during embryogenesis alters prenatal viability, intestinal function, the neurotransmitter complement, and the microbiome. Conditional deletion of choline acetyltransferase ( ChAT), the ACh synthetic enzyme, in neural crest-derived neurons ( ChAT-Null) was performed. Survival, ChAT activity, gut motility, and the microbiome were studied. ChAT was conditionally deleted in ENS neural crest-derived cells. Despite ChAT absence, mice were born live and survived the first 2 wk. They failed to gain significant weight in the third postnatal week, dying between postnatal d 18 and 30. Small intestinal transit of carmine red was 50% slower in ChAT-Nulls vs. WT and ChAT- Het. The colons of many neonatal ChAT-Null mice contained compacted feces, suggesting dysmotility. Microbiome analysis revealed dysbiosis in ChAT-Null mice. Developmental deletion of ChAT activity in enteric neurons results in proximal gastrointestinal tract dysmotility, critically diminished colonic transit, failure to thrive, intestinal dysbiosis, and death. ACh is necessary for sustained gut motility and survival of neonatal mice after weaning.-Johnson, C. D., Barlow-Anacker, A. J., Pierre, J. F., Touw, K., Erickson, C. S., Furness, J. B., Epstein, M. L., Gosain, A. Deletion of choline acetyltransferase in enteric neurons results in postnatal intestinal dysmotility and dysbiosis.
Keywords: acetylcholine; development; enteric nervous system; microbiome.
Conflict of interest statement
The authors thank Brian Torres for assistance in performing assays, Dr. Timur Mavlyutov (University of Wisconsin-Madison) for assistance with confocal imaging, and Dr. June Dahl (University of Wisconsin-Madison) for suggestions on the manuscript. This work was supported by U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grants K08DK098271, R03DK114543, and P30DK42086; by an American Pediatric Surgical Association Foundation Scholars Award (to A.G.); and by an American College of Surgeons George H.A. Clowes Career Development Award (to A.G.). The authors declare no conflicts of interest.
Figures

References
-
- Erickson C. S., Lee S. J., Barlow-Anacker A. J., Druckenbrod N. R., Epstein M. L., Gosain A. (2014) Appearance of cholinergic myenteric neurons during enteric nervous system development: comparison of different ChAT fluorescent mouse reporter lines. Neurogastroenterol. Motil. 26, 874–884 10.1111/nmo.12343 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

