Effects of Cytochrome P450 (CYP) 2C19 Genotypes on Steady-State Plasma Concentrations of Escitalopram and its Desmethyl Metabolite in Japanese Patients With Depression
- PMID: 29570504
- PMCID: PMC5959260
- DOI: 10.1097/FTD.0000000000000506
Effects of Cytochrome P450 (CYP) 2C19 Genotypes on Steady-State Plasma Concentrations of Escitalopram and its Desmethyl Metabolite in Japanese Patients With Depression
Abstract
Background: Plasma concentrations of the S-enantiomer of citalopram were different between extensive and poor CYP2C19 metabolizers in healthy subjects and depressed patients. However, most studies applied dose-corrected concentrations. Thus, we studied the effects of polymorphisms of the CYP2C19 gene on raw plasma drug concentrations in Japanese patients with depression.
Methods: Subjects in this study consisted of 412 depressed patients receiving 5, 10, 15, or 20 mg of escitalopram once a day. Plasma concentrations of escitalopram and desmethylescitalopram were quantified using HPLC. CYP2C19 genotypes were identified using polymerase chain reaction methods.
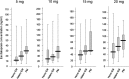
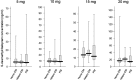
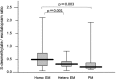
Results: There were no differences in the steady-state plasma concentrations of escitalopram or desmethylescitalopram in each dose group (5, 10, 15, or 20 mg of escitalopram) among CYP2C19 genotype groups. However, 1-way analysis of variance showed significant effects of CYP2C19 genotypes on the dose-adjusted plasma concentration of escitalopram but not in the dose-adjusted plasma concentration of desmethylescitalopram. Analysis of covariance including age, sex, and body weight showed significant effects of CYP2C19 genotypes on the dose-adjusted plasma concentration of escitalopram and the ratio of desmethylescitalopram to escitalopram.
Conclusions: These findings suggest that the CYP2C19 variants are associated with steady-state plasma concentrations of escitalopram to some extent but are not associated with desmethylescitalopram.
Conflict of interest statement
N. Yasui-Furukori has received grant/research support or honoraria from and has been on the panels of Dainippon, Mochida, MSD. The remaining authors declare no conflict of interest.
Figures

Similar articles
-
The Effects of Fluvoxamine on the Steady-State Plasma Concentrations of Escitalopram and Desmethylescitalopram in Depressed Japanese Patients.Ther Drug Monit. 2016 Aug;38(4):483-6. doi: 10.1097/FTD.0000000000000303. Ther Drug Monit. 2016. PMID: 27002781
-
CYP2C19 genotype predicts steady state escitalopram concentration in GENDEP.J Psychopharmacol. 2012 Mar;26(3):398-407. doi: 10.1177/0269881111414451. Epub 2011 Sep 17. J Psychopharmacol. 2012. PMID: 21926427 Clinical Trial.
-
Impact of age on serum concentrations of venlafaxine and escitalopram in different CYP2D6 and CYP2C19 genotype subgroups.Eur J Clin Pharmacol. 2014 Aug;70(8):933-40. doi: 10.1007/s00228-014-1696-8. Epub 2014 May 27. Eur J Clin Pharmacol. 2014. PMID: 24858822
-
The clinical pharmacokinetics of escitalopram.Clin Pharmacokinet. 2007;46(4):281-90. doi: 10.2165/00003088-200746040-00002. Clin Pharmacokinet. 2007. PMID: 17375980 Review.
-
Escitalopram Personalized Dosing: A Population Pharmacokinetics Repository Method.Drug Des Devel Ther. 2023 Sep 27;17:2955-2967. doi: 10.2147/DDDT.S425654. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37789969 Free PMC article. Review.
Cited by
-
Concentrations of escitalopram in blood of patients treated in a naturalistic setting: focus on patients with alcohol and benzodiazepine use disorder.Eur Arch Psychiatry Clin Neurosci. 2023 Feb;273(1):75-83. doi: 10.1007/s00406-022-01491-9. Epub 2022 Oct 7. Eur Arch Psychiatry Clin Neurosci. 2023. PMID: 36207527 Free PMC article.
-
Systematic review and meta-analysis on the therapeutic reference range for escitalopram: Blood concentrations, clinical effects and serotonin transporter occupancy.Front Psychiatry. 2022 Oct 17;13:972141. doi: 10.3389/fpsyt.2022.972141. eCollection 2022. Front Psychiatry. 2022. PMID: 36325531 Free PMC article.
-
Precision Medicine in Antidepressants Treatment.Handb Exp Pharmacol. 2023;280:131-186. doi: 10.1007/164_2023_654. Handb Exp Pharmacol. 2023. PMID: 37195310 Review.
-
The Effects of CYP2C19 Genotype on Proxies of SSRI Antidepressant Response in the UK Biobank.Pharmaceuticals (Basel). 2023 Sep 11;16(9):1277. doi: 10.3390/ph16091277. Pharmaceuticals (Basel). 2023. PMID: 37765085 Free PMC article.
-
Low Escitalopram Concentrations in Patients with Depression predict Treatment Failure: A Naturalistic Retrospective Study.Pharmacopsychiatry. 2023 Mar;56(2):73-80. doi: 10.1055/a-2039-2829. Epub 2023 Mar 21. Pharmacopsychiatry. 2023. PMID: 36944330 Free PMC article.
References
-
- Garnock-Jones KP, McCormack PL. Escitalopram: a review of its use in the management of major depressive disorder in adults. CNS Drugs. 2010;24:769–796. - PubMed
-
- Baldwin D, Woods R, Lawson R, et al. Efficacy of drug treatments for generalised anxiety disorder: systematic review and meta-analysis. BMJ. 2011;342:d1199. - PubMed
-
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–758. - PubMed
-
- Pastoor D, Gobburu J. Clinical pharmacology review of escitalopram for the treatment of depression. Expert Opin Drug Metab Toxicol. 2014;10:121–128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

