Micro-Economics of Apoptosis in Cancer: ncRNAs Modulation of BCL-2 Family Members
- PMID: 29570632
- PMCID: PMC5979352
- DOI: 10.3390/ijms19040958
Micro-Economics of Apoptosis in Cancer: ncRNAs Modulation of BCL-2 Family Members
Abstract
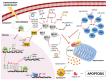
In the last few years, non-coding RNAs (ncRNAs) have been a hot topic in cancer research. Many ncRNAs were found to regulate the apoptotic process and to play a role in tumor cell resistance to treatment. The apoptotic program is on the frontline as self-defense from cancer onset, and evasion of apoptosis has been classified as one of the hallmarks of cancer responsible for therapy failure. The B-cell lymphoma 2 (BCL-2) family members are key players in the regulation of apoptosis and mediate the activation of the mitochondrial death machinery in response to radiation, chemotherapeutic agents and many targeted therapeutics. The balance between the pro-survival and the pro-apoptotic BCL-2 proteins is strictly controlled by ncRNAs. Here, we highlight the most common mechanisms exerted by microRNAs, long non-coding RNAs and circular RNAs on the main mediators of the intrinsic apoptotic cascade with particular focus on their significance in cancer biology.
Keywords: BCL-2 family; apoptosis; cancer; long non-coding RNAs; microRNAs; therapy resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

