Roles of Chloroplast Retrograde Signals and Ion Transport in Plant Drought Tolerance
- PMID: 29570668
- PMCID: PMC5979362
- DOI: 10.3390/ijms19040963
Roles of Chloroplast Retrograde Signals and Ion Transport in Plant Drought Tolerance
Abstract
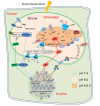
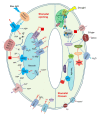
Worldwide, drought affects crop yields; therefore, understanding plants' strategies to adapt to drought is critical. Chloroplasts are key regulators of plant responses, and signals from chloroplasts also regulate nuclear gene expression during drought. However, the interactions between chloroplast-initiated retrograde signals and ion channels under stress are still not clear. In this review, we summarise the retrograde signals that participate in regulating plant stress tolerance. We compare chloroplastic transporters that modulate retrograde signalling through retrograde biosynthesis or as critical components in retrograde signalling. We also discuss the roles of important plasma membrane and tonoplast ion transporters that are involved in regulating stomatal movement. We propose how retrograde signals interact with ion transporters under stress.
Keywords: cotransporters; ion channels; pumps; retrograde signalling; stress responsive genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Evans A. The Feeding of the Nine Billion. [(accessed on 15 March 2018)]; Available online: http://www.wfp.org/stories/feeding-ten-billion-global-food-security-21st....
-
- FAO How to Feed the World in 2050. [(accessed on 15 March 2018)]; Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Fee....
-
- Zhang X., Cai X. Climate change impacts on global agricultural land availability. Environ. Res. Lett. 2011;6:014014. doi: 10.1088/1748-9326/6/1/014014. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

