Protective Effects of Gomisin N against Hepatic Cannabinoid Type 1 Receptor-Induced Insulin Resistance and Gluconeogenesis
- PMID: 29570673
- PMCID: PMC5979504
- DOI: 10.3390/ijms19040968
Protective Effects of Gomisin N against Hepatic Cannabinoid Type 1 Receptor-Induced Insulin Resistance and Gluconeogenesis
Abstract
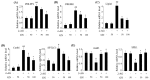
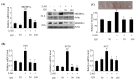
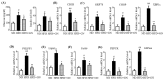
Activation of the hepatic cannabinoid type 1 receptor (CB1R) induces insulin resistance and gluconeogenesis via endoplasmic reticulum (ER) stress, thereby contributing to hyperglycemia. Gomisin N (GN) is a phytochemical derived from Schisandra chinensis. In the current study, we investigated the inhibitory effects of GN on hepatic CB1R-mediated insulin resistance and gluconeogenesis in 2-arachidonoylglycerol (AG; an agonist of CB1R)-treated HepG2 cells and in high-fat diet (HFD)-induced obese mice. Treatment with 2-AG induced the expression of ER stress markers, serine/threonine phosphatase PHLPP1, Lipin1, and ceramide synthesis genes, but reduced the expression of ceramide degradation genes in HepG2 cells. However, GN reversed 2-AG-mediated effects and improved the 2-AG-mediated impairment of insulin signaling. Furthermore, GN inhibited 2-AG-induced intracellular triglyceride accumulation and glucose production in HepG2 cells by downregulation of lipogenesis and gluconeogenesis genes, respectively. In vivo, GN administration to HFD obese mice reduced the HFD-induced increase in fasting blood glucose and insulin levels, which was accompanied with downregulation of HFD-induced expression of CB1R, ER stress markers, ceramide synthesis gene, and gluconeogenesis genes in the livers of HFD obese mice. These findings demonstrate that GN protects against hepatic CB1-mediated impairment of insulin signaling and gluconeogenesis, thereby contributing to the amelioration of hyperglycemia.
Keywords: cannabinoid type 1 receptor; endoplasmic reticulum stress; gluconeogenesis; gomisin N; insulin resistance; lipogenesis.
Conflict of interest statement
We declare that there is no conflict of interest.
Figures




References
-
- Biddinger S.B., Hernandez-Ono A., Rask-Madsen C., Haas J.T., Alemán J.Q., Suzuki R., Scapa E.F., Agarwal C., Carey M.C., Stephanopoulos G., et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. - DOI - PMC - PubMed
-
- Liu J., Zhou L., Xiong K., Godlewski G., Mukhopadhya B., Tam J., Yin S., Gao P., Shan X., Pickel J. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology. 2012;142:1218–1228. doi: 10.1053/j.gastro.2012.01.032. - DOI - PMC - PubMed
-
- Chanda D., Kim Y.H., Kim D.K., Lee M.W., Lee S.Y., Park T.S., Koo S.H., Lee C.H., Choi H.S. Activation of cannabinoid receptor type 1 (Cb1r) disrupts hepatic insulin receptor signaling via cyclic AMP-response element-binding protein H (Crebh)-mediated induction of Lipin1 gene. J. Biol. Chem. 2012;287:38041–38049. doi: 10.1074/jbc.M112.377978. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

