An Arabidopsis Clathrin Assembly Protein with a Predicted Role in Plant Defense Can Function as an Adenylate Cyclase
- PMID: 29570675
- PMCID: PMC6022867
- DOI: 10.3390/biom8020015
An Arabidopsis Clathrin Assembly Protein with a Predicted Role in Plant Defense Can Function as an Adenylate Cyclase
Abstract
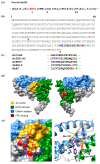
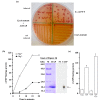
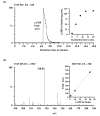
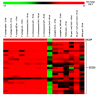
Adenylate cyclases (ACs), much like guanylate cyclases (GCs), are increasingly recognized as essential parts of many plant processes including biotic and abiotic stress responses. In order to identify novel ACs, we have applied a search motif derived from experimentally tested GCs and identified a number of Arabidopsis thaliana candidates including a clathrin assembly protein (AT1G68110; AtClAP). AtClAP contains a catalytic centre that can complement the AC-deficient mutant cyaA in E. coli, and a recombinant AtClAP fragment (AtClAP261-379) can produce cyclic adenosine 3',5' monophosphate (cAMP) from adenosine triphosphate (ATP) in vitro. Furthermore, an integrated analysis of gene expression and expression correlation implicate cAMP in pathogen defense and in actin cytoskeletal remodeling during endocytic internalization.
Keywords: Arabidopsis thaliana; adenylate cyclase; cAMP; clathrin assembly; endocytosis; pathogen responses.
Conflict of interest statement
The authors declare no competing interests with regard to contents of this article. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
Similar articles
-
The Arabidopsis thaliana K(+)-uptake permease 7 (AtKUP7) contains a functional cytosolic adenylate cyclase catalytic centre.FEBS Lett. 2015 Dec 21;589(24 Pt B):3848-52. doi: 10.1016/j.febslet.2015.11.038. Epub 2015 Nov 27. FEBS Lett. 2015. PMID: 26638082
-
An Arabidopsis thaliana leucine-rich repeat protein harbors an adenylyl cyclase catalytic center and affects responses to pathogens.J Plant Physiol. 2019 Jan;232:12-22. doi: 10.1016/j.jplph.2018.10.025. Epub 2018 Nov 3. J Plant Physiol. 2019. PMID: 30530199
-
Recombinant expression and functional testing of candidate adenylate cyclase domains.Methods Mol Biol. 2013;1016:13-25. doi: 10.1007/978-1-62703-441-8_2. Methods Mol Biol. 2013. PMID: 23681569
-
Cyclic Nucleotide Monophosphates in Plants and Plant Signaling.Handb Exp Pharmacol. 2017;238:87-103. doi: 10.1007/164_2015_35. Handb Exp Pharmacol. 2017. PMID: 26721677 Review.
-
Moonlighting Crypto-Enzymes and Domains as Ancient and Versatile Signaling Devices.Int J Mol Sci. 2024 Sep 2;25(17):9535. doi: 10.3390/ijms25179535. Int J Mol Sci. 2024. PMID: 39273482 Free PMC article. Review.
Cited by
-
Two triphosphate tunnel metalloenzymes from apple exhibit adenylyl cyclase activity.Front Plant Sci. 2022 Oct 6;13:992488. doi: 10.3389/fpls.2022.992488. eCollection 2022. Front Plant Sci. 2022. PMID: 36275530 Free PMC article.
-
Computational Identification of Functional Centers in Complex Proteins: A Step-by-Step Guide With Examples.Front Bioinform. 2021 Mar 25;1:652286. doi: 10.3389/fbinf.2021.652286. eCollection 2021. Front Bioinform. 2021. PMID: 36303732 Free PMC article.
-
Cross Talk Between Cyclic Nucleotides and Calcium Signaling Pathways in Plants-Achievements and Prospects.Front Plant Sci. 2021 Feb 16;12:643560. doi: 10.3389/fpls.2021.643560. eCollection 2021. Front Plant Sci. 2021. PMID: 33664763 Free PMC article. Review.
-
New Perspectives on Plant Adenylyl Cyclases.Front Mol Biosci. 2019 Dec 3;6:136. doi: 10.3389/fmolb.2019.00136. eCollection 2019. Front Mol Biosci. 2019. PMID: 31850369 Free PMC article.
-
The Arabidopsis thaliana K+-Uptake Permease 5 (AtKUP5) Contains a Functional Cytosolic Adenylate Cyclase Essential for K+ Transport.Front Plant Sci. 2018 Nov 13;9:1645. doi: 10.3389/fpls.2018.01645. eCollection 2018. Front Plant Sci. 2018. PMID: 30483296 Free PMC article.
References
-
- Marondedze C., Wong A., Thomas L., Irving H., Gehring C. Cyclic nucleotide monophosphates in plants and plant signaling. Handb. Exp. Pharmacol. 2017;238:87–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

