A Neural Circuit for the Suppression of Pain by a Competing Need State
- PMID: 29570993
- PMCID: PMC5877408
- DOI: 10.1016/j.cell.2018.02.057
A Neural Circuit for the Suppression of Pain by a Competing Need State
Abstract
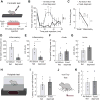
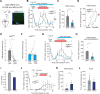
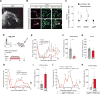
Hunger and pain are two competing signals that individuals must resolve to ensure survival. However, the neural processes that prioritize conflicting survival needs are poorly understood. We discovered that hunger attenuates behavioral responses and affective properties of inflammatory pain without altering acute nociceptive responses. This effect is centrally controlled, as activity in hunger-sensitive agouti-related protein (AgRP)-expressing neurons abrogates inflammatory pain. Systematic analysis of AgRP projection subpopulations revealed that the neural processing of hunger and inflammatory pain converge in the hindbrain parabrachial nucleus (PBN). Strikingly, activity in AgRP → PBN neurons blocked the behavioral response to inflammatory pain as effectively as hunger or analgesics. The anti-nociceptive effect of hunger is mediated by neuropeptide Y (NPY) signaling in the PBN. By investigating the intersection between hunger and pain, we have identified a neural circuit that mediates competing survival needs and uncovered NPY Y1 receptor signaling in the PBN as a target for pain suppression.
Keywords: AgRP neurons; analgesia; calcium imaging; hunger; inflammation; neuropeptide Y; nociception; optogenetics; pain; parabrachial nucleus.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures

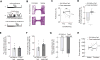


Comment in
-
Neural circuits: Balancing threats.Nat Rev Neurosci. 2018 May;19(5):254. doi: 10.1038/nrn.2018.35. Epub 2018 Apr 5. Nat Rev Neurosci. 2018. PMID: 29618806 No abstract available.
-
Hunger is a gatekeeper of pain in the brain.Nature. 2018 Apr;556(7702):445-446. doi: 10.1038/d41586-018-04759-0. Nature. 2018. PMID: 29686371 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

