The Autophagy Gene BcATG8 Regulates the Vegetative Differentiation and Pathogenicity of Botrytis cinerea
- PMID: 29572212
- PMCID: PMC5960959
- DOI: 10.1128/AEM.02455-17
The Autophagy Gene BcATG8 Regulates the Vegetative Differentiation and Pathogenicity of Botrytis cinerea
Abstract
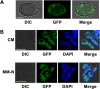
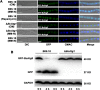
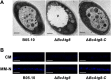
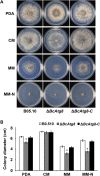
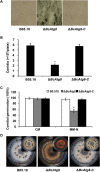
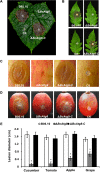
Autophagy is a conserved degradation process that maintains intracellular homeostasis to ensure normal cell differentiation and development in eukaryotes. ATG8 is one of the key molecular components of the autophagy pathway. In this study, we identified and characterized BcATG8, a homologue of Saccharomyces cerevisiae (yeast) ATG8 in the necrotrophic plant pathogen Botrytis cinerea Yeast complementation experiments demonstrated that BcATG8 can functionally complement the defects of the yeast ATG8 null mutant. Direct physical interaction between BcAtg8 and BcAtg4 was detected in the yeast two-hybrid system. Subcellular localization assays showed that green fluorescent protein-tagged BcAtg8 (GFP-BcAtg8) localized in the cytoplasm as preautophagosomal structures (PAS) under general conditions but mainly accumulated in the lumen of vacuoles in the case of autophagy induction. Deletion of BcATG8 (ΔBcAtg8 mutant) blocked autophagy and significantly impaired mycelial growth, conidiation, sclerotial formation, and virulence. In addition, the conidia of the ΔBcAtg8 mutant contained fewer lipid droplets (LDs), and quantitative real-time PCR (qRT-PCR) assays revealed that the basal expression levels of the LD metabolism-related genes in the mutant were significantly different from those in the wild-type (WT) strain. All of these phenotypic defects were restored by gene complementation. These results indicate that BcATG8 is essential for autophagy to regulate fungal development, pathogenesis, and lipid metabolism in B. cinereaIMPORTANCE The gray mold fungus Botrytis cinerea is an economically important plant pathogen with a broad host range. Although there are fungicides for its control, many classes of fungicides have failed due to its genetic plasticity. Exploring the fundamental biology of B. cinerea can provide the theoretical basis for sustainable and long-term disease management. Autophagy is an intracellular process for degradation and recycling of cytosolic materials in eukaryotes and is now known to be vital for fungal life. Here, we report studies of the biological role of the autophagy gene BcATG8 in B. cinerea The results suggest that autophagy plays a crucial role in vegetative differentiation and virulence of B. cinerea.
Keywords: BcATG8; Botrytis cinerea; autophagy; vegetative differentiation; virulence.
Copyright © 2018 American Society for Microbiology.
Figures


Similar articles
-
The autophagy-related gene BcATG1 is involved in fungal development and pathogenesis in Botrytis cinerea.Mol Plant Pathol. 2017 Feb;18(2):238-248. doi: 10.1111/mpp.12396. Epub 2016 Jul 1. Mol Plant Pathol. 2017. PMID: 26972592 Free PMC article.
-
Involvement of the cysteine protease BcAtg4 in development and virulence of Botrytis cinerea.Curr Genet. 2019 Feb;65(1):293-300. doi: 10.1007/s00294-018-0882-0. Epub 2018 Aug 30. Curr Genet. 2019. PMID: 30167777
-
Ubiquitin-like activating enzymes BcAtg3 and BcAtg7 participate in development and pathogenesis of Botrytis cinerea.Curr Genet. 2018 Aug;64(4):919-930. doi: 10.1007/s00294-018-0810-3. Epub 2018 Feb 7. Curr Genet. 2018. PMID: 29417220
-
Botrytis cinerea virulence factors: new insights into a necrotrophic and polyphageous pathogen.FEMS Microbiol Lett. 2007 Dec;277(1):1-10. doi: 10.1111/j.1574-6968.2007.00930.x. FEMS Microbiol Lett. 2007. PMID: 17986079 Review.
-
Recent Advances in the Study of the Plant Pathogenic Fungus Botrytis cinerea and its Interaction with the Environment.Curr Protein Pept Sci. 2017;18(10):976-989. doi: 10.2174/1389203717666160809160915. Curr Protein Pept Sci. 2017. PMID: 27526927 Review.
Cited by
-
The MAP Kinase PvMK1 Regulates Hyphal Development, Autophagy, and Pathogenesis in the Bayberry Twig Blight Fungus Pestalotiopsis versicolor.J Fungi (Basel). 2023 May 24;9(6):606. doi: 10.3390/jof9060606. J Fungi (Basel). 2023. PMID: 37367542 Free PMC article.
-
PlAtg8-mediated autophagy regulates vegetative growth, sporangial cleavage, and pathogenesis in Peronophythora litchii.Microbiol Spectr. 2024 Jan 11;12(1):e0353123. doi: 10.1128/spectrum.03531-23. Epub 2023 Dec 12. Microbiol Spectr. 2024. PMID: 38084976 Free PMC article.
-
Regulation of Tomato Fruit Autophagic Flux and Promotion of Fruit Ripening by the Autophagy-Related Gene SlATG8f.Plants (Basel). 2023 Sep 21;12(18):3339. doi: 10.3390/plants12183339. Plants (Basel). 2023. PMID: 37765504 Free PMC article.
-
Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1.Autophagy. 2021 Jan;17(1):1-382. doi: 10.1080/15548627.2020.1797280. Epub 2021 Feb 8. Autophagy. 2021. PMID: 33634751 Free PMC article.
-
The FpPPR1 Gene Encodes a Pentatricopeptide Repeat Protein That Is Essential for Asexual Development, Sporulation, and Pathogenesis in Fusarium pseudograminearum.Front Genet. 2021 Jan 15;11:535622. doi: 10.3389/fgene.2020.535622. eCollection 2020. Front Genet. 2021. PMID: 33584782 Free PMC article.
References
-
- Pande S, Galloway J, Gaur PM, Siddique KHM, Tripathi HS, Taylor P, MacLeod MWJ, Basandrai AK, Bakr A, Joshi S, Krishna Kishore G, Isenegger DA, Narayana Rao J, Sharma M. 2006. Botrytis grey mould of chickpea: a review of biology, epidemiology, and disease management. Aust J Agric Res 57:1137–1150. doi:10.1071/AR06120. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

