Convergence and Divergence of CRH Amacrine Cells in Mouse Retinal Circuitry
- PMID: 29572434
- PMCID: PMC5895998
- DOI: 10.1523/JNEUROSCI.2518-17.2018
Convergence and Divergence of CRH Amacrine Cells in Mouse Retinal Circuitry
Abstract
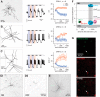
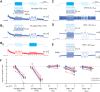
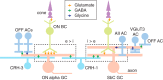
Inhibitory interneurons sculpt the outputs of excitatory circuits to expand the dynamic range of information processing. In mammalian retina, >30 types of amacrine cells provide lateral inhibition to vertical, excitatory bipolar cell circuits, but functional roles for only a few amacrine cells are well established. Here, we elucidate the function of corticotropin-releasing hormone (CRH)-expressing amacrine cells labeled in Cre-transgenic mice of either sex. CRH cells costratify with the ON alpha ganglion cell, a neuron highly sensitive to positive contrast. Electrophysiological and optogenetic analyses demonstrate that two CRH types (CRH-1 and CRH-3) make GABAergic synapses with ON alpha cells. CRH-1 cells signal via graded membrane potential changes, whereas CRH-3 cells fire action potentials. Both types show sustained ON-type responses to positive contrast over a range of stimulus conditions. Optogenetic control of transmission at CRH-1 synapses demonstrates that these synapses are tuned to low temporal frequencies, maintaining GABA release during fast hyperpolarizations during brief periods of negative contrast. CRH amacrine cell output is suppressed by prolonged negative contrast, when ON alpha ganglion cells continue to receive inhibitory input from converging OFF-pathway amacrine cells; the converging ON- and OFF-pathway inhibition balances tonic excitatory drive to ON alpha cells. Previously, it was demonstrated that CRH-1 cells inhibit firing by suppressed-by-contrast (SbC) ganglion cells during positive contrast. Therefore, divergent outputs of CRH-1 cells inhibit two ganglion cell types with opposite responses to positive contrast. The opposing responses of ON alpha and SbC ganglion cells are explained by differing excitation/inhibition balance in the two circuits.SIGNIFICANCE STATEMENT A goal of neuroscience research is to explain the function of neural circuits at the level of specific cell types. Here, we studied the function of specific types of inhibitory interneurons, corticotropin-releasing hormone (CRH) amacrine cells, in the mouse retina. Genetic tools were used to identify and manipulate CRH cells, which make GABAergic synapses with a well studied ganglion cell type, the ON alpha cell. CRH cells converge with other types of amacrine cells to tonically inhibit ON alpha cells and balance their high level of excitation. CRH cells diverge to different types of ganglion cell, the unique properties of which depend on their balance of excitation and inhibition.
Keywords: GABA; amacrine cell; corticotropin-releasing hormone; mouse retina; optogenetics; retinal ganglion cell.
Copyright © 2018 the authors 0270-6474/18/383753-14$15.00/0.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
