A neuronal basis for fear discrimination in the lateral amygdala
- PMID: 29572443
- PMCID: PMC5865209
- DOI: 10.1038/s41467-018-03682-2
A neuronal basis for fear discrimination in the lateral amygdala
Abstract
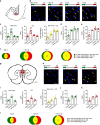
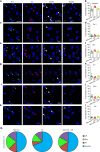
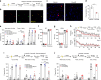
In the presence of new stimuli, it is crucial for survival to react with defensive responses in the presence of stimuli that resemble threats but also to not react with defensive behavior in response to new harmless stimuli. Here, we show that in the presence of new uncertain stimuli with sensory features that produce an ambiguous interpretation, discriminative processes engage a subset of excitatory and inhibitory neurons within the lateral amygdala (LA) that are partially different from those engaged by fear processes. Inducing the pharmacogenetic deletion of this neuronal ensemble caused fear generalization but left anxiety-like response, fear memory and extinction processes intact. These data reveal that two opposite neuronal processes account for fear discrimination and generalization within the LA and suggest a potential pathophysiological mechanism for the impaired discrimination that characterizes fear-related disorders.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Pavlov, I. P. Conditioned Reflexes (Oxford University Press, London, 1927).
-
- Ghirlanda S, Enquist MA. A century of generalization. Anim. Behav. 2003;66:15–36. doi: 10.1006/anbe.2003.2174. - DOI

