Biocatalytic Oxidation Reactions: A Chemist's Perspective
- PMID: 29573076
- PMCID: PMC6099261
- DOI: 10.1002/anie.201800343
Biocatalytic Oxidation Reactions: A Chemist's Perspective
Abstract
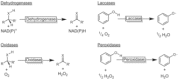
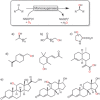
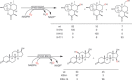
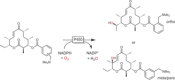
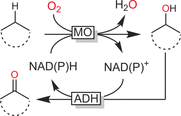
Oxidation chemistry using enzymes is approaching maturity and practical applicability in organic synthesis. Oxidoreductases (enzymes catalysing redox reactions) enable chemists to perform highly selective and efficient transformations ranging from simple alcohol oxidations to stereoselective halogenations of non-activated C-H bonds. For many of these reactions, no "classical" chemical counterpart is known. Hence oxidoreductases open up shorter synthesis routes based on a more direct access to the target products. The generally very mild reaction conditions may also reduce the environmental impact of biocatalytic reactions compared to classical counterparts. In this Review, we critically summarise the most important recent developments in the field of biocatalytic oxidation chemistry and identify the most pressing bottlenecks as well as promising solutions.
Keywords: Baeyer-Villiger oxidation; biocatalysis; halogenation; oxidation; oxyfunctionalisation.
© 2018 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures











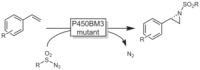
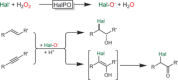




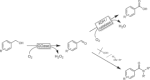

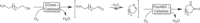
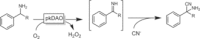


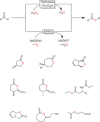

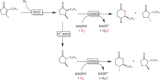
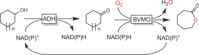



References
-
- None
-
- Dijkman W., Gonzalo G., Mattevi A., Fraaije M., Appl. Microbiol. Biotechnol. 2013, 97, 5177–5188; - PubMed
-
- Pedersen A. T., Birmingham W. R., Rehn G., Charnock S. J., Turner N. J., Woodley J. M., Org. Process Res. Dev. 2015, 19, 1580–1589.
-
- Siebum A., van Wijk A., Schoevaart R., Kieboom T., J. Mol. Catal. B 2006, 41, 141–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

