Phase Ib trial of folate binding protein (FBP)-derived peptide vaccines, E39 and an attenuated version, E39': An analysis of safety and immune response
- PMID: 29574039
- PMCID: PMC5988975
- DOI: 10.1016/j.clim.2018.03.010
Phase Ib trial of folate binding protein (FBP)-derived peptide vaccines, E39 and an attenuated version, E39': An analysis of safety and immune response
Abstract
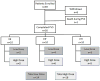
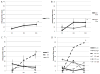
In this randomized phase Ib trial, we tested combining the E39 peptide vaccine with a vaccine created from E39', an attenuated version of E39. Patients with breast or ovarian cancer, who were disease-free after standard of care therapy, were enrolled and randomized to one of three arms. Arm EE received six E39 inoculations; arm EE' received three E39 inoculations followed by three E39'; and arm E'E received three E39' inoculations, followed by three E39. Within each arm, the first five patients received 500 μg of peptide and the remainder received 1000 μg. Patients were followed for toxicity, and immune responses were measured. This initial analysis after completion of the primary vaccination series has confirmed the safety of both vaccines. Immune analyses suggest incorporating the attenuated version of the peptide improves immune responses and that sequencing of E39 followed by E39' might produce the optimal immune response.
Trial registration: NCT02019524.
Keywords: Activation induced cell death; Attenuated vaccine; Breast cancer; Folate binding protein; Ovarian cancer; Peptide vaccine.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr. George Peoples is a consultant to Galena Biopharma and has partial inventor rights for the E39 and E39′ vaccines.
The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of San Antonio Military Medical Center, the U.S. Army Medical Department, U.S. Air Force Medical Department, the Department of the Army, the Department of Air Force, Department of Defense or the U.S. Government.
Figures

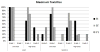


References
-
- Burstein HJ, Krilov L, Aragon-Ching JB, Baxter NN, Chiorean EG, Chow WA, et al. Clinical Cancer Advances 2017: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J Clin Oncol. 2017;35:1341–67. - PubMed
-
- Vreeland TJ, Clifton GT, Herbert GS, Hale DF, Jackson DO, Berry JS, et al. Gaining ground on a cure through synergy: combining checkpoint inhibitors with cancer vaccines. Expert Rev Clin Immunol. 2016;12:1347–57. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

