Modeling Short QT Syndrome Using Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes
- PMID: 29574456
- PMCID: PMC5907581
- DOI: 10.1161/JAHA.117.007394
Modeling Short QT Syndrome Using Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes
Abstract
Background: Short QT syndrome (SQTS), a disorder associated with characteristic ECG QT-segment abbreviation, predisposes affected patients to sudden cardiac death. Despite some progress in assessing the organ-level pathophysiology and genetic changes of the disorder, the understanding of the human cellular phenotype and discovering of an optimal therapy has lagged because of a lack of appropriate human cellular models of the disorder. The objective of this study was to establish a cellular model of SQTS using human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs).
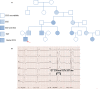
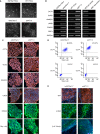
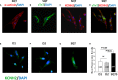
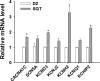
Methods and results: This study recruited 1 patient with short QT syndrome type 1 carrying a mutation (N588K) in KCNH2 as well as 2 healthy control subjects. We generated hiPSCs from their skin fibroblasts, and differentiated hiPSCs into cardiomyocytes (hiPSC-CMs) for physiological and pharmacological studies. The hiPSC-CMs from the patient showed increased rapidly activating delayed rectifier potassium channel current (IKr) density and shortened action potential duration compared with healthy control hiPSC-CMs. Furthermore, they demonstrated abnormal calcium transients and rhythmic activities. Carbachol increased the arrhythmic events in SQTS but not in control cells. Gene and protein expression profiling showed increased KCNH2 expression in SQTS cells. Quinidine but not sotalol or metoprolol prolonged the action potential duration and abolished arrhythmic activity induced by carbachol.
Conclusions: Patient-specific hiPSC-CMs are able to recapitulate single-cell phenotype features of SQTS and provide novel opportunities to further elucidate the cellular disease mechanism and test drug effects.
Keywords: arrhythmia (heart rhythm disorders); arrhythmia (mechanisms); ion channel; short QT syndrome.
© 2018 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures




References
-
- Guerrier K, Kwiatkowski D, Czosek RJ, Spar DS, Anderson JB, Knilans TK. Short QT interval prevalence and clinical outcomes in a pediatric population. Circ Arrhythm Electrophysiol. 2015;8:1460–1464. - PubMed
-
- Priori SG, Blomstrom‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez‐Madrid A, Nikolaou N, Norekval TM, Spaulding C, Van Veldhuisen DJ. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–2867. - PubMed
-
- Giustetto C, Di Monte F, Wolpert C, Borggrefe M, Schimpf R, Sbragia P, Leone G, Maury P, Anttonen O, Haissaguerre M, Gaita F. Short QT syndrome: clinical findings and diagnostic‐therapeutic implications. Eur Heart J. 2006;27:2440–2447. - PubMed
-
- Gaita F, Giustetto C, Bianchi F, Wolpert C, Schimpf R, Riccardi R, Grossi S, Richiardi E, Borggrefe M. Short QT syndrome: a familial cause of sudden death. Circulation. 2003;108:965–970. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

