Dimethyl fumarate (DMF) vs. monoethyl fumarate (MEF) salts for the treatment of plaque psoriasis: a review of clinical data
- PMID: 29574575
- PMCID: PMC6060759
- DOI: 10.1007/s00403-018-1825-9
Dimethyl fumarate (DMF) vs. monoethyl fumarate (MEF) salts for the treatment of plaque psoriasis: a review of clinical data
Abstract
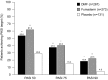
Fumarates (fumaric acid esters, FAEs) are orally administered systemic agents used for the treatment of psoriasis and multiple sclerosis. In 1994, a proprietary combination of FAEs was licensed for psoriasis by the German Drug Administration for use within Germany. Since then, fumarates have been established as one of the most commonly used treatments for moderate-to-severe psoriasis in Germany and other countries. The licensed FAE formulation contains dimethyl fumarate (DMF), as well as calcium, zinc, and magnesium salts of monoethyl fumarate (MEF). While the clinical efficacy of this FAE mixture is well established, the combination of esters on which it is based, and its dosing regimen, was determined empirically. Since the mid-1990s, the modes of action and contribution of the different FAEs to their overall therapeutic effect in psoriasis, as well as their adverse event profile, have been investigated in more detail. In this article, the available clinical data for DMF are reviewed and compared with data for the other FAEs. The current evidence substantiates that DMF is the main active compound, via its metabolic transformation to monomethyl fumarate (MMF). A recent phase III randomized and placebo-controlled trial including more than 700 patients demonstrated therapeutic equivalence when comparing the licensed FAE combination with DMF alone, in terms of psoriasis clearance according to the Psoriasis Area and Severity Index (PASI) and Physician's Global Assessment (PGA). Thus, DMF as monotherapy for the treatment of psoriasis is as efficacious as in combination with MEF, making the addition of such fumarate derivatives unnecessary.
Keywords: DMF; Dimethyl fumarate; Fumaric acid esters; MEF; Monoethyl fumarate; Psoriasis.
Conflict of interest statement
Conflict of interest
LL has no conflicts of interest to declare. KA is a stock holder and former employee of Bayer AG and has received financial support and/or served as consultant, advisory board member, or speaker for AbbVie, Antabio, Almirall, EmertiPharma, Galderma, Leo, Loreal, Eli Lilly, and Novartis. IPC and AA are employees of Almirall S.A. UM has been an advisor and/or received speakers honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Almirall, Amgen, Biogen, Boehringer-Ingelheim, Celgene, Dr. Reddy’s, Eli Lilly, Foamix, Formycon, Forward Pharma, Janssen, Leo Pharma, Medac, MSD, Novartis, VBL and Xenoport.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Figures
References
-
- American Academy of Dermatology Work Group. Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Sect. 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65:137–174. doi: 10.1016/j.jaad.2010.11.055. - DOI - PubMed
Related articles recently published in Archives of Dermatological Research (selected by the journal’s editorial staff)
-
- Augustin M, Eissing L, Langenbruch A, Enk A, Luger T, Maassen D, Mrowietz U, Reich K, Reusch M, Stromer K, Thaci D, von Kiedrowski R, Radtke MA. The German National Program on Psoriasis Health Care 2005–2015: results and experiences. Arch Dermatol Res. 2016;308:389–400. doi: 10.1007/s00403-016-1637-8. - DOI - PMC - PubMed
-
- Florek AG, Wang CJ, Armstrong AW. Treatment preferences and treatment satisfaction among psoriasis patients: a systematic review. Arch Dermatol Res. 2018 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

