Dosing of Ceftriaxone and Metronidazole for Children With Severe Acute Malnutrition
- PMID: 29574688
- PMCID: PMC6282491
- DOI: 10.1002/cpt.1078
Dosing of Ceftriaxone and Metronidazole for Children With Severe Acute Malnutrition
Abstract
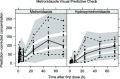
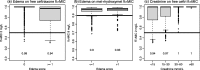
Infants and young children with severe acute malnutrition (SAM) are treated with empiric broad-spectrum antimicrobials. Parenteral ceftriaxone is currently a second-line agent for invasive infection. Oral metronidazole principally targets small intestinal bacterial overgrowth. Children with SAM may have altered drug absorption, distribution, metabolism, and elimination. Population pharmacokinetics of ceftriaxone and metronidazole were studied, with the aim of recommending optimal dosing. Eighty-one patients with SAM (aged 2-45 months) provided 234 postdose pharmacokinetic samples for total ceftriaxone, metronidazole, and hydroxymetronidazole. Ceftriaxone protein binding was also measured in 190 of these samples. A three-compartment model adequately described free ceftriaxone, with a Michaelis-Menten model for concentration and albumin-dependent protein binding. A one-compartment model was used for both metronidazole and hydroxymetronidazole, with only 1% of hydroxymetronidazole predicted to be formed during first-pass. Simulations showed 80 mg/kg once daily of ceftriaxone and 12.5 mg/kg twice daily of metronidazole were sufficient to reach therapeutic targets.
© 2018 The Authors Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures



References
-
- Williams, C. M. & Berkley, J. A. severe acute malnutrition update: Current WHO guidelines and the WHO essential medicine list for children. J. Clin. Pharmacol. <http://www.who.int/selection_medicines/committees/expert/21/applications...> (2016).
-
- Organization, W.H. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Childhood Illnesses. (World Health Organization, 2013). <https://books.google.co.uk/books?id=nSSgnQAACAAJ> - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

