Enantioselective Catalyst Systems from Copper(II) Triflate and BINOL-Silanediol
- PMID: 29575279
- PMCID: PMC5996771
- DOI: 10.1002/chem.201801304
Enantioselective Catalyst Systems from Copper(II) Triflate and BINOL-Silanediol
Abstract
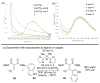
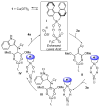
Silanediol and copper catalysis are merged, for the first time, to create an enhanced Lewis acid catalyst system for enantioselective heterocycle functionalization. The promise of this silanediol and copper catalyst combination is demonstrated in the enantioselective addition of indoles to alkylidene malonates to give rise to the desirable adducts in excellent yield and high enantiomeric excess. From these studies, 1,1'-bi-2-naphthol (BINOL)-based silanediols emerge as one-of-a-kind cocatalysts. Their potential role in the reaction pathway is also discussed.
Keywords: Friedel-Crafts reactions; cooperative catalysis; copper; enantioselectivity; silanediols.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
-
For reviews of silanediols, see: Wieting JM, Hardman-Baldwin AM, Visco MD, Mattson AE. Aldrichimica Acta. 2016;49:15–20.Franz AK, Wilson SO. J Med Chem. 2013;56:388.Sieburth SM, Chen CA. Eur J Org Chem. 2006:311.Min GK, Hernandez D, Skrydstrup T. Acc Chem Res. 2013;46:457.
-
-
-
For examples of the medicinal applications of silanediols, see: Sieburth SM, Nittoli T, Mutahi A, Guo L. Angew Chem Int Ed. 1998;37:812.Angew Chem. 1998;110:845.Mutahi MW, Nittoli T, Guo L, Sieburth SM. J Am Chem Soc. 2002;124:7363.
-
-
-
For an application of silanediol molecular recognition in sensing, see: Kondo S, Bie Y, Yamamura M. Org Lett. 2013;15:520–523.
-
-
-
For selected examples of silanediols in achiral catalysis, see: Tran NT, Min T, Franz AK. Chem Eur J. 2011;17:9897.Schafer AG, Wieting JM, Mattson AE. Org Lett. 2011;13:5228–5232.Hardman-Baldwin AM, Mattson AE. ChemSusChem. 2014;7:3275–3278.
-
-
-
For an early report of silanediols in anion recognition, see: Kondo S, Harada T, Tanaka R, Unno M. Org Lett. 2006;8:4621–4624.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

