Site-Selective Cysteine-Cyclooctyne Conjugation
- PMID: 29575377
- PMCID: PMC6150453
- DOI: 10.1002/anie.201800860
Site-Selective Cysteine-Cyclooctyne Conjugation
Abstract
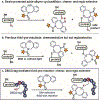
We report a site-selective cysteine-cyclooctyne conjugation reaction between a seven-residue peptide tag (DBCO-tag, Leu-Cys-Tyr-Pro-Trp-Val-Tyr) at the N or C terminus of a peptide or protein and various aza-dibenzocyclooctyne (DBCO) reagents. Compared to a cysteine peptide control, the DBCO-tag increases the rate of the thiol-yne reaction 220-fold, thereby enabling selective conjugation of DBCO-tag to DBCO-linked fluorescent probes, affinity tags, and cytotoxic drug molecules. Fusion of DBCO-tag with the protein of interest enables regioselective cysteine modification on proteins that contain multiple endogenous cysteines; these examples include green fluorescent protein and the antibody trastuzumab. This study demonstrates that short peptide tags can aid in accelerating bond-forming reactions that are often slow to non-existent in water.
Keywords: bioconjugation; bioorthogonal chemistry; cysteine-cyclooctyne reaction; dibenzocyclooctyne; protein modification.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

