No effects without causes: the Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases
- PMID: 29575574
- PMCID: PMC6055827
- DOI: 10.1111/brv.12407
No effects without causes: the Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases
Abstract
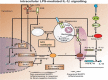
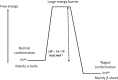
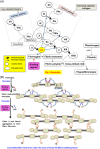
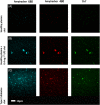
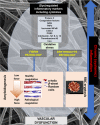
Since the successful conquest of many acute, communicable (infectious) diseases through the use of vaccines and antibiotics, the currently most prevalent diseases are chronic and progressive in nature, and are all accompanied by inflammation. These diseases include neurodegenerative (e.g. Alzheimer's, Parkinson's), vascular (e.g. atherosclerosis, pre-eclampsia, type 2 diabetes) and autoimmune (e.g. rheumatoid arthritis and multiple sclerosis) diseases that may appear to have little in common. In fact they all share significant features, in particular chronic inflammation and its attendant inflammatory cytokines. Such effects do not happen without underlying and initially 'external' causes, and it is of interest to seek these causes. Taking a systems approach, we argue that these causes include (i) stress-induced iron dysregulation, and (ii) its ability to awaken dormant, non-replicating microbes with which the host has become infected. Other external causes may be dietary. Such microbes are capable of shedding small, but functionally significant amounts of highly inflammagenic molecules such as lipopolysaccharide and lipoteichoic acid. Sequelae include significant coagulopathies, not least the recently discovered amyloidogenic clotting of blood, leading to cell death and the release of further inflammagens. The extensive evidence discussed here implies, as was found with ulcers, that almost all chronic, infectious diseases do in fact harbour a microbial component. What differs is simply the microbes and the anatomical location from and at which they exert damage. This analysis offers novel avenues for diagnosis and treatment.
Keywords: LPS; amplification; amyloid; blood clotting; inflammation; iron dysregulation.
© 2018 The Authors. Biological Reviews published by John Wiley & Sons Ltd on behalf of Cambridge Philosophical Society.
Figures
Similar articles
-
Iron Dysregulation and Dormant Microbes as Causative Agents for Impaired Blood Rheology and Pathological Clotting in Alzheimer's Type Dementia.Front Neurosci. 2018 Nov 16;12:851. doi: 10.3389/fnins.2018.00851. eCollection 2018. Front Neurosci. 2018. PMID: 30519157 Free PMC article. Review.
-
Correlative Light-Electron Microscopy detects lipopolysaccharide and its association with fibrin fibres in Parkinson's Disease, Alzheimer's Disease and Type 2 Diabetes Mellitus.Sci Rep. 2018 Nov 14;8(1):16798. doi: 10.1038/s41598-018-35009-y. Sci Rep. 2018. PMID: 30429533 Free PMC article.
-
Iron Dysregulation and Inflammagens Related to Oral and Gut Health Are Central to the Development of Parkinson's Disease.Biomolecules. 2020 Dec 29;11(1):30. doi: 10.3390/biom11010030. Biomolecules. 2020. PMID: 33383805 Free PMC article. Review.
-
A Dormant Microbial Component in the Development of Preeclampsia.Front Med (Lausanne). 2016 Nov 29;3:60. doi: 10.3389/fmed.2016.00060. eCollection 2016. Front Med (Lausanne). 2016. PMID: 27965958 Free PMC article. Review.
-
A Bacterial Component to Alzheimer's-Type Dementia Seen via a Systems Biology Approach that Links Iron Dysregulation and Inflammagen Shedding to Disease.J Alzheimers Dis. 2016 Jun 18;53(4):1237-56. doi: 10.3233/JAD-160318. J Alzheimers Dis. 2016. PMID: 27340854 Free PMC article. Review.
Cited by
-
Covid-19: The Rollercoaster of Fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes.Int J Mol Sci. 2020 Jul 21;21(14):5168. doi: 10.3390/ijms21145168. Int J Mol Sci. 2020. PMID: 32708334 Free PMC article. Review.
-
Association of serum ferritin with metabolic syndrome in eight cities in China.Food Sci Nutr. 2020 Feb 3;8(3):1406-1414. doi: 10.1002/fsn3.1408. eCollection 2020 Mar. Food Sci Nutr. 2020. PMID: 32180950 Free PMC article.
-
Platelet activity and hypercoagulation in type 2 diabetes.Cardiovasc Diabetol. 2018 Nov 2;17(1):141. doi: 10.1186/s12933-018-0783-z. Cardiovasc Diabetol. 2018. PMID: 30388964 Free PMC article.
-
The impact of aging and oxidative stress in metabolic and nervous system disorders: programmed cell death and molecular signal transduction crosstalk.Front Immunol. 2023 Nov 8;14:1273570. doi: 10.3389/fimmu.2023.1273570. eCollection 2023. Front Immunol. 2023. PMID: 38022638 Free PMC article. Review.
-
TMBIM4 Deficiency Facilitates NLRP3 Inflammasome Activation-Induced Pyroptosis of Trophoblasts: A Potential Pathogenesis of Preeclampsia.Biology (Basel). 2023 Jan 29;12(2):208. doi: 10.3390/biology12020208. Biology (Basel). 2023. PMID: 36829486 Free PMC article.
References
-
- Afzal, S. , Bojesen, S. E. & Nordestgaard, B. G. (2013). Low 25‐hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clinical Chemistry 59, 381–391. - PubMed
-
- Afzal, S. , Bojesen, S. E. & Nordestgaard, B. G. (2014). Reduced 25‐hydroxyvitamin D and risk of Alzheimer's disease and vascular dementia. Alzheimer's & Dementia 10, 296–302. - PubMed
-
- Afzal, S. , Brøndum‐Jacobsen, P. , Bojesen, S. E. & Nordestgaard, B. G. (2014c). Vitamin D concentration, obesity, and risk of diabetes: a Mendelian randomisation study. Lancet Diabetes & Endocrinology 2, 298–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

