A neurosteroid analogue with T-type calcium channel blocking properties is an effective hypnotic, but is not harmful to neonatal rat brain
- PMID: 29576117
- PMCID: PMC6200096
- DOI: 10.1016/j.bja.2017.12.039
A neurosteroid analogue with T-type calcium channel blocking properties is an effective hypnotic, but is not harmful to neonatal rat brain
Abstract
Background: More than 4 million children are exposed annually to sedatives and general anaesthetics (GAs) in the USA alone. Recent data suggest that common GAs can be detrimental to brain development causing neurodegeneration and long-term cognitive impairments. Challenged by a recent US Food and Drug Administration (FDA) warning about potentially neurotoxic effects of GAs in children, there is an urgent need to develop safer GAs.
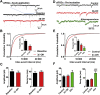
Methods: Postnatal Day 7 (P7) rat pups of both sexes were exposed to six (repeated every 2 h) injections of equipotent hypnotic doses of ketamine or the neuroactive steroid (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH) for 12 h. Loss of righting reflex was used to assess hypnotic properties and therapeutic index; quantitative caspase-3 immunohistochemistry was used to assess developmental neuroapoptosis; patch-clamp recordings in acute brain slices were used to assess the effects of 3β-OH on neuronal excitability and synaptic transmission. Cognitive abilities of rats exposed to ketamine, 3β-OH, or vehicle at P7 were assessed in young adulthood using the radial arm maze.
Results: The neuroactive steroid 3β-OH has a therapeutic index similar to ketamine, a commonly used clinical GA. We report that 3β-OH is safe and, unlike ketamine, does not cause neuroapoptosis or impair cognitive development when administered to P7 rat pups. Interestingly, 3β-OH blocks T-type calcium channels and presynaptically dampens synaptic transmission at hypnotically-relevant brain concentrations, but it lacks a direct effect on γ-aminobutyric acid A or glutamate-gated ion channels.
Conclusions: The neurosteroid 3β-OH is a relatively safe hypnotic that warrants further consideration for paediatric anaesthesia.
Keywords: calcium channels; developmental neurotoxicity; neurosteroid.
Copyright © 2018 British Journal of Anaesthesia. Published by Elsevier Ltd. All rights reserved.
Figures





Comment in
-
Quest for new drugs: a way to solve anaesthesia neurotoxicity?Br J Anaesth. 2018 Apr;120(4):619-621. doi: 10.1016/j.bja.2018.01.024. Epub 2018 Feb 24. Br J Anaesth. 2018. PMID: 29576101 No abstract available.
References
-
- Loepke A.W., Istaphanous G.K., McAuliffe J.J., 3rd The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009;108:90–104. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

