Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies
- PMID: 29576375
- PMCID: PMC5904288
- DOI: 10.1016/j.ccell.2018.02.010
Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies
Abstract
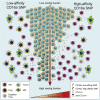
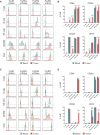
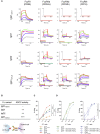
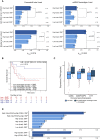
With the use of a mouse model expressing human Fc-gamma receptors (FcγRs), we demonstrated that antibodies with isotypes equivalent to ipilimumab and tremelimumab mediate intra-tumoral regulatory T (Treg) cell depletion in vivo, increasing the CD8+ to Treg cell ratio and promoting tumor rejection. Antibodies with improved FcγR binding profiles drove superior anti-tumor responses and survival. In patients with advanced melanoma, response to ipilimumab was associated with the CD16a-V158F high affinity polymorphism. Such activity only appeared relevant in the context of inflamed tumors, explaining the modest response rates observed in the clinical setting. Our data suggest that the activity of anti-CTLA-4 in inflamed tumors may be improved through enhancement of FcγR binding, whereas poorly infiltrated tumors will likely require combination approaches.
Keywords: CTLA-4; Fc-gamma receptors; IgG subclass; antibody-dependent cell-mediated cytotoxicity; immune checkpoints; immune regulatory antibodies; ipilimumab; regulatory T cell depletion; tremelimumab; tumor immunotherapy.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
More Rules, Still Exceptions: Understanding Immunomodulatory Antibody Activity In Vivo.Cancer Cell. 2018 Apr 9;33(4):545-546. doi: 10.1016/j.ccell.2018.03.016. Cancer Cell. 2018. PMID: 29634942
References
-
- Bruhns P., Iannascoli B., England P., Mancardi D.A., Fernandez N., Jorieux S., Daëron M. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. - PubMed
-
- Bulliard Y., Jolicoeur R., Zhang J., Dranoff G., Wilson N.S., Brogdon J.L. OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy. Immunol. Cell Biol. 2014;92:475–480. - PubMed
-
- Cartron G., Dacheux L., Salles G., Solal-Celigny P., Bardos P., Colombat P., Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- C11496/A17786/CRUK_/Cancer Research UK/United Kingdom
- 18176/CRUK_/Cancer Research UK/United Kingdom
- 19278/CRUK_/Cancer Research UK/United Kingdom
- 22246/CRUK_/Cancer Research UK/United Kingdom
- 20764/CRUK_/Cancer Research UK/United Kingdom
- C5759/A20465/CRUK_/Cancer Research UK/United Kingdom
- 21999/CRUK_/Cancer Research UK/United Kingdom
- C416/A18088/CRUK_/Cancer Research UK/United Kingdom
- MC_UP_1203/1/MRC_/Medical Research Council/United Kingdom
- 17786/CRUK_/Cancer Research UK/United Kingdom
- 20276/CRUK_/Cancer Research UK/United Kingdom
- DH_/Department of Health/United Kingdom
- 20265/CRUK_/Cancer Research UK/United Kingdom
- C33499/A20265/CRUK_/Cancer Research UK/United Kingdom
- MR/P014712/1/MRC_/Medical Research Council/United Kingdom
- C36463/A22246/CRUK_/Cancer Research UK/United Kingdom
- 16463/CRUK_/Cancer Research UK/United Kingdom
- C36463/A20764/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

