Dermatologic Toxicity Occurring During Anti-EGFR Monoclonal Inhibitor Therapy in Patients With Metastatic Colorectal Cancer: A Systematic Review
- PMID: 29576427
- PMCID: PMC6773267
- DOI: 10.1016/j.clcc.2017.12.004
Dermatologic Toxicity Occurring During Anti-EGFR Monoclonal Inhibitor Therapy in Patients With Metastatic Colorectal Cancer: A Systematic Review
Abstract
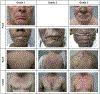
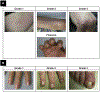
Monoclonal antibody inhibitors of the epidermal growth factor receptor (EGFR) have been shown to improve outcomes for patients with metastatic colorectal cancer (mCRC) without RAS gene mutations. However, treatment with anti-EGFR agents can be associated with toxicities of the skin, nails, hair, and eyes. Because these dermatologic toxicities can result in treatment discontinuation and affect patient quality of life, their management is an important focus when administering anti-EGFR monoclonal antibodies. The present systematic review describes the current data reporting the nature and incidence of, and management and treatment options for, dermatologic toxicities occurring during anti-EGFR treatment of mCRC. A search of the National Library of Medicine PubMed database from January 1, 2009, to August 18, 2016, identified relevant reports discussing dermatologic toxicity management among patients with mCRC receiving anti-EGFR therapy. The studies were grouped by type and rated by level of evidence using the GRADE approach developed by the Agency for Healthcare Research and Quality. Overall, 269 reports were reviewed (nonrandomized trials, n = 120; randomized trials, n = 31; retrospective studies, n = 15; reviews, n = 39). Dermatologic toxicity of any grade occurs in most patients who receive anti-EGFR therapy; approximately 10% to 20% of patients experienced grade 3/4 toxicity. The most common dermatologic toxicities include papulopustular/acneiform rash, xerosis, and pruritus; however, nail changes, hair abnormalities, and ocular conditions also occur. Guidance for managing these toxicities includes the use of inexpensive emollient ointments and moisturizers, avoidance of sun exposure, avoidance of irritants, and the use of short showers. Several studies also found that preemptive treatment was more effective than reactive treatment at limiting the incidence and severity of skin toxicity. With appropriate treatment, the dermatologic toxicities associated with anti-EGFR monoclonal antibody therapy can be managed, minimizing patient discomfort and the need for therapy interruption and/or discontinuation. Additionally, preemptive treatment can reduce dermatologic toxicity severity, ultimately yielding better quality of life.
Keywords: Dermatologic toxicity management; Epidermal growth factor receptor; Patient outcomes; Skin toxicity.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Management and grading of EGFR inhibitor-induced cutaneous toxicity.Future Oncol. 2018 Oct;14(24):2531-2541. doi: 10.2217/fon-2018-0187. Epub 2018 May 4. Future Oncol. 2018. PMID: 29727211 Review.
-
Current Recommendations and Novel Strategies for the Management of Skin Toxicities Related to Anti-EGFR Therapies in Patients with Metastatic Colorectal Cancer.Clin Drug Investig. 2019 Sep;39(9):825-834. doi: 10.1007/s40261-019-00811-7. Clin Drug Investig. 2019. PMID: 31264159 Review.
-
Pre-emptive skin toxicity treatment for anti-EGFR drugs: evaluation of efficacy of skin moisturizers and lymecycline. A phase II study.Support Care Cancer. 2013 Jun;21(6):1691-5. doi: 10.1007/s00520-012-1715-1. Epub 2013 Jan 13. Support Care Cancer. 2013. PMID: 23314653 Clinical Trial.
-
Predictors of Tumor Response to Cetuximab and Panitumumab in 116 Patients and a Review of Approaches to Managing Skin Toxicity.Actas Dermosifiliogr. 2015 Jul-Aug;106(6):483-92. doi: 10.1016/j.ad.2015.01.006. Epub 2015 Mar 19. Actas Dermosifiliogr. 2015. PMID: 25798804 English, Spanish.
-
Risk factor analysis for anti-epidermal growth factor receptor monoclonal antibody-induced skin toxicities in real-world metastatic colorectal cancer treatment.Support Care Cancer. 2023 Aug 2;31(8):504. doi: 10.1007/s00520-023-07973-3. Support Care Cancer. 2023. PMID: 37528282
Cited by
-
Navigating metastatic colorectal treatment options in the USA: a survey of patient acceptance of skin toxicities associated with Vectibix.Support Care Cancer. 2021 Nov;29(11):6731-6740. doi: 10.1007/s00520-021-06134-8. Epub 2021 May 11. Support Care Cancer. 2021. PMID: 33973081 Free PMC article.
-
Skin Toxicity as a Predictor of Survival in Metastatic Colorectal Cancer Patients Treated with Anti-EGFR: Fact or Fallacy?Cancers (Basel). 2023 Mar 8;15(6):1663. doi: 10.3390/cancers15061663. Cancers (Basel). 2023. PMID: 36980549 Free PMC article.
-
Surface-engineered bacteria in drug development.Microb Biotechnol. 2024 Oct;17(10):e70033. doi: 10.1111/1751-7915.70033. Microb Biotechnol. 2024. PMID: 39403960 Free PMC article. Review.
-
miR-887-3p Inhibits the Progression of Colorectal Cancer via Downregulating DNMT1 Expression and Regulating P53 Expression.Comput Intell Neurosci. 2022 Jun 27;2022:7179733. doi: 10.1155/2022/7179733. eCollection 2022. Comput Intell Neurosci. 2022. Retraction in: Comput Intell Neurosci. 2023 Dec 13;2023:9831724. doi: 10.1155/2023/9831724. PMID: 35795731 Free PMC article. Retracted.
-
Dual Erb B Inhibition in Oesophago-gastric Cancer (DEBIOC): A phase I dose escalating safety study and randomised dose expansion of AZD8931 in combination with oxaliplatin and capecitabine chemotherapy in patients with oesophagogastric adenocarcinoma.Eur J Cancer. 2020 Jan;124:131-141. doi: 10.1016/j.ejca.2019.10.010. Epub 2019 Nov 22. Eur J Cancer. 2020. PMID: 31765988 Free PMC article.
References
-
- Weng W, Feng J, Qin H, et al. Molecular therapy of colorectal cancer: progress and future directions. Int J Cancer 2015; 136:493–502. - PubMed
-
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007; 25:1658–64. - PubMed
-
- Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009; 27:663–71. - PubMed
-
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009; 360:1408–17. - PubMed
-
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010; 28: 4697–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

