Common PIEZO1 Allele in African Populations Causes RBC Dehydration and Attenuates Plasmodium Infection
- PMID: 29576450
- PMCID: PMC5889333
- DOI: 10.1016/j.cell.2018.02.047
Common PIEZO1 Allele in African Populations Causes RBC Dehydration and Attenuates Plasmodium Infection
Abstract
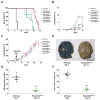
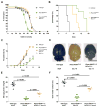
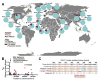
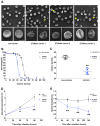
Hereditary xerocytosis is thought to be a rare genetic condition characterized by red blood cell (RBC) dehydration with mild hemolysis. RBC dehydration is linked to reduced Plasmodium infection in vitro; however, the role of RBC dehydration in protection against malaria in vivo is unknown. Most cases of hereditary xerocytosis are associated with gain-of-function mutations in PIEZO1, a mechanically activated ion channel. We engineered a mouse model of hereditary xerocytosis and show that Plasmodium infection fails to cause experimental cerebral malaria in these mice due to the action of Piezo1 in RBCs and in T cells. Remarkably, we identified a novel human gain-of-function PIEZO1 allele, E756del, present in a third of the African population. RBCs from individuals carrying this allele are dehydrated and display reduced Plasmodium infection in vitro. The existence of a gain-of-function PIEZO1 at such high frequencies is surprising and suggests an association with malaria resistance.
Keywords: PIEZO1; cerebral malaria; dehydration; functional variants; genomics; human genetics; ion channel; malaria; mechanotransduction; red blood cell.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, Fénéant-Thibault M, Tertian G, de Jaureguiberry JP, Syfuss PY, et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun. 2013;4:1884. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

