Mammalian Oocytes Locally Remodel Follicular Architecture to Provide the Foundation for Germline-Soma Communication
- PMID: 29576478
- PMCID: PMC5882553
- DOI: 10.1016/j.cub.2018.02.039
Mammalian Oocytes Locally Remodel Follicular Architecture to Provide the Foundation for Germline-Soma Communication
Abstract
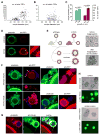
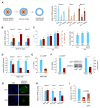
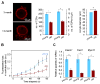
Germ cells develop in a microenvironment created by the somatic cells of the gonad [1-3]. Although in males, the germ and somatic support cells lie in direct contact, in females, a thick extracellular coat surrounds the oocyte, physically separating it from the somatic follicle cells [4]. To bypass this barrier to communication, narrow cytoplasmic extensions of the follicle cells traverse the extracellular coat to reach the oocyte plasma membrane [5-9]. These delicate structures provide the sole platform for the contact-mediated communication between the oocyte and its follicular environment that is indispensable for production of a fertilizable egg [8, 10-15]. Identifying the mechanisms underlying their formation should uncover conserved regulators of fertility. We show here in mice that these structures, termed transzonal projections (TZPs), are specialized filopodia whose number amplifies enormously as oocytes grow, enabling increased germ-soma communication. By creating chimeric complexes of genetically tagged oocytes and follicle cells, we demonstrate that follicle cells elaborate new TZPs that push through the extracellular coat to reach the oocyte surface. We further show that growth-differentiation factor 9, produced by the oocyte, drives the formation of new TZPs, uncovering a key yet unanticipated role for the germ cell in building these essential bridges of communication. Moreover, TZP number and germline-soma communication are strikingly reduced in reproductively aged females. Thus, the growing oocyte locally remodels follicular architecture to ensure that its developmental needs are met, and an inability of somatic follicle cells to respond appropriately to oocyte-derived cues may contribute to human infertility.
Keywords: fertility; filopodia; follicle; intercellular signaling; oocyte.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no competing interests
Figures

Comment in
-
Reproduction: Oocytes Call, Granulosa Cells Connect.Curr Biol. 2018 Apr 23;28(8):R354-R356. doi: 10.1016/j.cub.2018.03.005. Curr Biol. 2018. PMID: 29689210
References
-
- El-Hayek S, Clarke HJ. Control of oocyte growth and development by intercellular communication within the follicular niche. Res Probl Cell Differ. 2016;58:191–224. - PubMed
-
- Wassarman PM, Litscher ES. Biogenesis of the mouse egg’s extracellular coat, the zona pellucida. Curr Top Dev Biol. 2013;102:243–266. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

