Immunomodulatory Effects of Diterpene Quinone Derivatives from the Roots of Horminum pyrenaicum in Human PBMC
- PMID: 29576845
- PMCID: PMC5821946
- DOI: 10.1155/2018/2980295
Immunomodulatory Effects of Diterpene Quinone Derivatives from the Roots of Horminum pyrenaicum in Human PBMC
Abstract
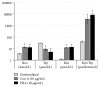
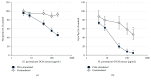
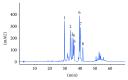
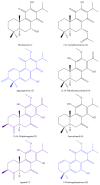
Several phytochemicals were shown to interfere with redox biology in the human system. Moreover, redox biochemistry is crucially involved in the orchestration of immunological cascades. When screening for immunomodulatory compounds, the two interferon gamma- (IFN-γ-) dependent immunometabolic pathways of tryptophan breakdown via indoleamine 2,3-dioxygenase-1 (IDO-1) and neopterin formation by GTP-cyclohydrolase 1 (GTP-CH-I) represent prominent targets, as IFN-γ-related signaling is strongly sensitive to oxidative triggers. Herein, the analysis of these pathway activities in human peripheral mononuclear cells was successfully applied in a bioactivity-guided fractionation strategy to screen for anti-inflammatory substances contained in the root of Horminum (H.) pyrenaicum L. (syn. Dragon's mouth), the only representative of the monophyletic genus Horminum. Four abietane diterpene quinone derivatives (horminone, 7-O-acetylhorminone, inuroyleanol and its 15,16-dehydro-derivative, a novel natural product), two nor-abietane diterpene quinones (agastaquinone and 3-deoxyagastaquinone) and two abeo 18 (4 → 3) abietane diterpene quinones (agastol and its 15,16-dehydro-derivative) could be identified. These compounds were able to dose-dependently suppress the above mentioned pathways with different potency. Beside the description of new active compounds, this study demonstrates the feasibility of integrating IDO-1 and GTP-CH-I activity in the search for novel anti-inflammatory compounds, which can then be directed towards a more detailed mode of action analysis.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

