Palmitoylethanolamide Dampens Reactive Astrogliosis and Improves Neuronal Trophic Support in a Triple Transgenic Model of Alzheimer's Disease: In Vitro and In Vivo Evidence
- PMID: 29576849
- PMCID: PMC5822864
- DOI: 10.1155/2018/4720532
Palmitoylethanolamide Dampens Reactive Astrogliosis and Improves Neuronal Trophic Support in a Triple Transgenic Model of Alzheimer's Disease: In Vitro and In Vivo Evidence
Abstract
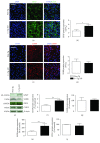
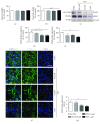
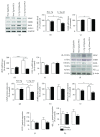
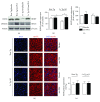
Alzheimer's disease (AD) is a neurodegenerative disorder responsible for the majority of dementia cases in elderly people. It is widely accepted that the main hallmarks of AD are not only senile plaques and neurofibrillary tangles but also reactive astrogliosis, which often precedes detrimental deposits and neuronal atrophy. Such phenomenon facilitates the regeneration of neural networks; however, under some circumstances, like in AD, reactive astrogliosis is detrimental, depriving neurons of the homeostatic support, thus contributing to neuronal loss. We investigated the presence of reactive astrogliosis in 3×Tg-AD mice and the effects of palmitoylethanolamide (PEA), a well-documented anti-inflammatory molecule, by in vitro and in vivo studies. In vitro results revealed a basal reactive state in primary cortical 3×Tg-AD-derived astrocytes and the ability of PEA to counteract such phenomenon and improve viability of 3×Tg-AD-derived neurons. In vivo observations, performed using ultramicronized- (um-) PEA, a formulation endowed with best bioavailability, confirmed the efficacy of this compound. Moreover, the schedule of treatment, mimicking the clinic use (chronic daily administration), revealed its beneficial pharmacological properties in dampening reactive astrogliosis and promoting the glial neurosupportive function. Collectively, our results encourage further investigation on PEA effects, suggesting it as an alternative or adjunct treatment approach for innovative AD therapy.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

