Beneficial Effects of Human Mesenchymal Stromal Cells on Porcine Hepatocyte Viability and Albumin Secretion
- PMID: 29577046
- PMCID: PMC5822000
- DOI: 10.1155/2018/1078547
Beneficial Effects of Human Mesenchymal Stromal Cells on Porcine Hepatocyte Viability and Albumin Secretion
Abstract
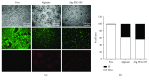
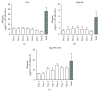
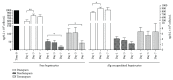
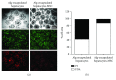
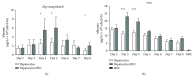
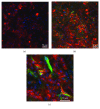
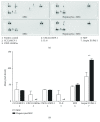
Porcine hepatocytes transplanted during acute liver failure might support metabolic functions until the diseased liver recovers its function. Here, we isolated high numbers of viable pig hepatocytes and evaluated hepatocyte functionality after encapsulation. We further investigated whether coculture and coencapsulation of hepatocytes with human multipotent mesenchymal stromal cells (MSC) are beneficial on hepatocyte function. Livers from 10 kg pigs (n = 9) were harvested, and hepatocytes were isolated from liver suspensions for microencapsulation using alginate and poly(ethylene-glycol)- (PEG-) grafted alginate hydrogels, either alone or in combination with MSC. Viability, albumin secretion, and diazepam catabolism of hepatocytes were measured for one week. 9.2 ± 3.6 × 109 hepatocytes with 95.2 ± 3.1% viability were obtained after isolation. At day 3, free hepatocytes displayed 99% viability, whereas microencapsulation in alginate and PEG-grafted alginate decreased viability to 62% and 48%, respectively. Albumin secretion and diazepam catabolism occurred in free and microencapsulated hepatocytes. Coencapsulation of hepatocytes with MSC significantly improved viability and albumin secretion at days 4 and 8 (p < 0.05). Coculture with MSC significantly increased and prolonged albumin secretion. In conclusion, we established a protocol for isolation and microencapsulation of high numbers of viable pig hepatocytes and demonstrated that the presence of MSC is beneficial for the viability and function of porcine hepatocytes.
Figures

Similar articles
-
A comprehensive review of advances in hepatocyte microencapsulation: selecting materials and preserving cell viability.Front Immunol. 2024 Apr 17;15:1385022. doi: 10.3389/fimmu.2024.1385022. eCollection 2024. Front Immunol. 2024. PMID: 38694507 Free PMC article.
-
Microencapsulation of Hepatocytes and Mesenchymal Stem Cells for Therapeutic Applications.Methods Mol Biol. 2017;1506:259-271. doi: 10.1007/978-1-4939-6506-9_18. Methods Mol Biol. 2017. PMID: 27830559
-
Alginate microencapsulated hepatocytes optimised for transplantation in acute liver failure.PLoS One. 2014 Dec 1;9(12):e113609. doi: 10.1371/journal.pone.0113609. eCollection 2014. PLoS One. 2014. PMID: 25438038 Free PMC article.
-
Coencapsulation of hepatocytes with bone marrow mesenchymal stem cells improves hepatocyte-specific functions.Transplantation. 2009 Nov 27;88(10):1178-85. doi: 10.1097/TP.0b013e3181bc288b. Transplantation. 2009. PMID: 19935371
-
Inventing Engineered Organoids for end-stage liver failure patients.J Mol Histol. 2022 Aug;53(4):611-621. doi: 10.1007/s10735-022-10085-7. Epub 2022 Jul 27. J Mol Histol. 2022. PMID: 35882727 Free PMC article. Review.
Cited by
-
The Crosstalk between Mesenchymal Stromal/Stem Cells and Hepatocytes in Homeostasis and under Stress.Int J Mol Sci. 2023 Oct 16;24(20):15212. doi: 10.3390/ijms242015212. Int J Mol Sci. 2023. PMID: 37894893 Free PMC article. Review.
-
A comprehensive review of advances in hepatocyte microencapsulation: selecting materials and preserving cell viability.Front Immunol. 2024 Apr 17;15:1385022. doi: 10.3389/fimmu.2024.1385022. eCollection 2024. Front Immunol. 2024. PMID: 38694507 Free PMC article.
-
Engineered Liver Tissue Culture in an In Vitro Tubular Perfusion System.Tissue Eng Part A. 2020 Dec;26(23-24):1369-1377. doi: 10.1089/ten.TEA.2020.0213. Epub 2020 Nov 24. Tissue Eng Part A. 2020. PMID: 33054685 Free PMC article.
-
Direct Differentiation of Human Embryonic Stem Cells to 3D Functional Hepatocyte-like Cells in Alginate Microencapsulation Sphere.Cells. 2022 Oct 5;11(19):3134. doi: 10.3390/cells11193134. Cells. 2022. PMID: 36231094 Free PMC article.
-
Contributions of Europeans to Xenotransplantation Research: 2. Pig Islet and Cell Xenotransplantation.Transpl Int. 2025 Apr 17;38:14143. doi: 10.3389/ti.2025.14143. eCollection 2025. Transpl Int. 2025. PMID: 40313362 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

