Selectable high-yield recombinant protein production in human cells using a GFP/YFP nanobody affinity support
- PMID: 29577475
- PMCID: PMC5980532
- DOI: 10.1002/pro.3409
Selectable high-yield recombinant protein production in human cells using a GFP/YFP nanobody affinity support
Abstract
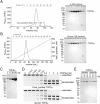
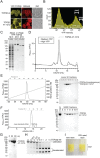
Recombinant protein expression systems that produce high yields of pure proteins and multi-protein complexes are essential to meet the needs of biologists, biochemists, and structural biologists using X-ray crystallography and cryo-electron microscopy. An ideal expression system for recombinant human proteins is cultured human cells where the correct translation and chaperone machinery are present. However, compared to bacterial expression systems, human cell cultures present several technical challenges to their use as an expression system. We developed a method that utilizes a YFP fusion-tag to generate recombinant proteins using suspension-cultured HEK293F cells. YFP is a dual-function tag that enables direct visualization and fluorescence-based selection of high expressing clones for and rapid purification using a high-stringency, high-affinity anti-GFP/YFP nanobody support. We demonstrate the utility of this system by expressing two large human proteins, TOP2α (340 KDa dimer) and a TOP2β catalytic core (260 KDa dimer). This robustly and reproducibly yields >10 mg/L liter of cell culture using transient expression or 2.5 mg/L using stable expression.
Keywords: FACS; GFP-tag; HEK293F; Topoisomerase 2; YFP-tag; nanobody; recombinant protein expression.
Published 2018. This article is a US Government work and is in the public domain in the USA.
Figures


Similar articles
-
High-efficiency recombinant protein purification using mCherry and YFP nanobody affinity matrices.Protein Sci. 2022 Sep;31(9):e4383. doi: 10.1002/pro.4383. Protein Sci. 2022. PMID: 36040252 Free PMC article.
-
Recombinant Topoisomerase 2 Production Using Cultured Human Cell Lines.Methods Mol Biol. 2025;2928:207-222. doi: 10.1007/978-1-0716-4550-5_17. Methods Mol Biol. 2025. PMID: 40372648
-
Engineering and characterization of GFP-targeting nanobody: Expression, purification, and post-translational modification analysis.Protein Expr Purif. 2024 Sep;221:106501. doi: 10.1016/j.pep.2024.106501. Epub 2024 May 21. Protein Expr Purif. 2024. PMID: 38782081
-
A nanobody:GFP bacterial platform that enables functional enzyme display and easy quantification of display capacity.Microb Cell Fact. 2016 May 3;15:71. doi: 10.1186/s12934-016-0474-y. Microb Cell Fact. 2016. PMID: 27142225 Free PMC article.
-
Use of the Nanofitin Alternative Scaffold as a GFP-Ready Fusion Tag.PLoS One. 2015 Nov 5;10(11):e0142304. doi: 10.1371/journal.pone.0142304. eCollection 2015. PLoS One. 2015. PMID: 26539718 Free PMC article.
Cited by
-
Structure and function of H+/K+ pump mutants reveal Na+/K+ pump mechanisms.Nat Commun. 2022 Sep 9;13(1):5270. doi: 10.1038/s41467-022-32793-0. Nat Commun. 2022. PMID: 36085139 Free PMC article.
-
Mapping the global interactome of the ARF family reveals spatial organization in cellular signaling pathways.J Cell Sci. 2024 May 1;137(9):jcs262140. doi: 10.1242/jcs.262140. Epub 2024 May 14. J Cell Sci. 2024. PMID: 38606629 Free PMC article.
-
Easily Established and Multifunctional Synthetic Nanobody Libraries as Research Tools.Int J Mol Sci. 2022 Jan 27;23(3):1482. doi: 10.3390/ijms23031482. Int J Mol Sci. 2022. PMID: 35163405 Free PMC article. Review.
-
High-efficiency recombinant protein purification using mCherry and YFP nanobody affinity matrices.Protein Sci. 2022 Sep;31(9):e4383. doi: 10.1002/pro.4383. Protein Sci. 2022. PMID: 36040252 Free PMC article.
-
Branched ubiquitin chain binding and deubiquitination by UCH37 facilitate proteasome clearance of stress-induced inclusions.Elife. 2021 Nov 11;10:e72798. doi: 10.7554/eLife.72798. Elife. 2021. PMID: 34761751 Free PMC article.
References
-
- Contreras‐Gómez A, Sánchez‐Mirón A, García‐Camacho F, Molina‐Grima E, Chisti Y (2014) Protein production using the baculovirus‐insect cell expression system. Biotechnol Prog 30:1–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

