Streptococcus dysgalactiae subsp. dysgalactiae isolated from milk of the bovine udder as emerging pathogens: In vitro and in vivo infection of human cells and zebrafish as biological models
- PMID: 29577680
- PMCID: PMC6341033
- DOI: 10.1002/mbo3.623
Streptococcus dysgalactiae subsp. dysgalactiae isolated from milk of the bovine udder as emerging pathogens: In vitro and in vivo infection of human cells and zebrafish as biological models
Abstract
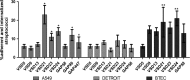
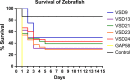
Streptococcus dysgalactiae subsp. dysgalactiae (SDSD) is a major cause of bovine mastitis and has been regarded as an animal-restricted pathogen, although rare infections have been described in humans. Previous studies revealed the presence of virulence genes encoded by phages of the human pathogen Group A Streptococcus pyogenes (GAS) in SDSD isolated from the milk of bovine udder with mastitis. The isolates SDSD VSD5 and VSD13 could adhere and internalize human primary keratinocyte cells, suggesting a possible human infection potential of bovine isolates. In this work, the in vitro and in vivo potential of SDSD to internalize/adhere human cells of the respiratory track and zebrafish as biological models was evaluated. Our results showed that, in vitro, bovine SDSD strains could interact and internalize human respiratory cell lines and that this internalization was dependent on an active transport mechanism and that, in vivo, SDSD are able to cause invasive infections producing zebrafish morbidity and mortality. The infectious potential of these isolates showed to be isolate-specific and appeared to be independent of the presence or absence of GAS phage-encoded virulence genes. Although the infection ability of the bovine SDSD strains was not as strong as the human pathogenic S. pyogenes in the zebrafish model, results suggested that these SDSD isolates are able to interact with human cells and infect zebrafish, a vertebrate infectious model, emerging as pathogens with zoonotic capability.
Keywords: Streptococcus dysgalactiae subsp. dysgalactiae; bovine; host adhesion/internalization; systemic infection; zebrafish.
© 2018 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Figures



Similar articles
-
New Insights on Streptococcus dysgalactiae subsp. dysgalactiae Isolates.Front Microbiol. 2021 Jul 15;12:686413. doi: 10.3389/fmicb.2021.686413. eCollection 2021. Front Microbiol. 2021. PMID: 34335512 Free PMC article.
-
Assessing in vivo and in vitro biofilm development by Streptococcus dysgalactiae subsp. dysgalactiae using a murine model of catheter-associated biofilm and human keratinocyte cell.Front Cell Infect Microbiol. 2022 Jul 19;12:874694. doi: 10.3389/fcimb.2022.874694. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35928206 Free PMC article.
-
Infection of human keratinocytes by Streptococcus dysgalactiae subspecies dysgalactiae isolated from milk of the bovine udder.Microbes Infect. 2016 Apr;18(4):290-3. doi: 10.1016/j.micinf.2015.11.005. Epub 2015 Dec 1. Microbes Infect. 2016. PMID: 26655883
-
Potential virulence factors of Streptococcus dysgalactiae associated with bovine mastitis.Vet Microbiol. 1998 Mar 15;61(1-2):93-110. doi: 10.1016/s0378-1135(98)00172-2. Vet Microbiol. 1998. PMID: 9646469 Review.
-
Potential factors involved in the early pathogenesis of Streptococcus uberis mastitis: a review.Folia Microbiol (Praha). 2021 Aug;66(4):509-523. doi: 10.1007/s12223-021-00879-9. Epub 2021 Jun 3. Folia Microbiol (Praha). 2021. PMID: 34085166 Review.
Cited by
-
Comparative Genomic Analysis of Streptococcus dysgalactiae subspecies dysgalactiae Isolated From Bovine Mastitis in China.Front Microbiol. 2021 Oct 22;12:751863. doi: 10.3389/fmicb.2021.751863. eCollection 2021. Front Microbiol. 2021. PMID: 34745056 Free PMC article.
-
Diversity of Streptococcus spp. and genomic characteristics of Streptococcus uberis isolated from clinical mastitis of cattle in Bangladesh.Front Vet Sci. 2023 Jul 18;10:1198393. doi: 10.3389/fvets.2023.1198393. eCollection 2023. Front Vet Sci. 2023. PMID: 37533458 Free PMC article.
-
Structural insights of an LCP protein-LytR-from Streptococcus dysgalactiae subs. dysgalactiae through biophysical and in silico methods.Front Chem. 2024 Aug 6;12:1379914. doi: 10.3389/fchem.2024.1379914. eCollection 2024. Front Chem. 2024. PMID: 39170866 Free PMC article.
-
Phylogenetic analysis and accessory genome diversity reveal insight into the evolutionary history of Streptococcus dysgalactiae.Front Microbiol. 2022 Jul 19;13:952110. doi: 10.3389/fmicb.2022.952110. eCollection 2022. Front Microbiol. 2022. PMID: 35928143 Free PMC article.
-
New Insights on Streptococcus dysgalactiae subsp. dysgalactiae Isolates.Front Microbiol. 2021 Jul 15;12:686413. doi: 10.3389/fmicb.2021.686413. eCollection 2021. Front Microbiol. 2021. PMID: 34335512 Free PMC article.
References
-
- Abdelsalam, M. , Asheg, A. , & Eissa, A. E. (2013). Streptococcus dysgalactiae: An emerging pathogen of fishes and mammals. International Journal of Veterinary Science and Medicine, 1, 1–6. 10.1016/j.ijvsm.2013.04.002 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

