RND transporters in the living world
- PMID: 29577985
- PMCID: PMC6151166
- DOI: 10.1016/j.resmic.2018.03.001
RND transporters in the living world
Abstract
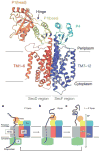
Transporters of the RND superfamily are well-known as the major drug efflux pumps of Gram-negative bacteria. However, they are widespread in organisms ranging from Archaea to Eukaryotes, and perform diverse functions. This review gives a brief overview of these diverse members of the superfamily with emphasis on their structure and functions.
Keywords: Phylogeny; Quaternary structure; Superfamily.
Copyright © 2018 Institut Pasteur. Published by Elsevier Masson SAS. All rights reserved.
Figures



References
-
- Yen MR, Chen JS, Marquez JL, Sun EI, Saier MH. Multidrug resistance: phylogenetic characterization of superfamilies of secondary carriers that include drug exporters. Methods Mol Biol. 2010;637:47–64. - PubMed
-
- Tseng TT, Gratwick KS, Kollman J, Park D, Nies DH, Goffeau A, et al. The RND permease superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. J Mol Microbiol Biotechnol. 1999;1:107–25. - PubMed
-
- Pos KM. Drug transport mechanism of the AcrB efflux pump. Biochim Biophys Acta. 2009;1794:782–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

