Angiotensin-converting enzyme in innate and adaptive immunity
- PMID: 29578208
- PMCID: PMC6192041
- DOI: 10.1038/nrneph.2018.15
Angiotensin-converting enzyme in innate and adaptive immunity
Abstract
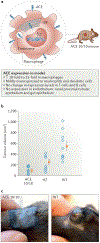
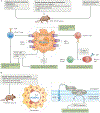
Angiotensin-converting enzyme (ACE) - a zinc-dependent dicarboxypeptidase with two catalytic domains - plays a major part in blood pressure regulation by converting angiotensin I to angiotensin II. However, ACE cleaves many peptides besides angiotensin I and thereby affects diverse physiological functions, including renal development and male reproduction. In addition, ACE has a role in both innate and adaptive responses by modulating macrophage and neutrophil function - effects that are magnified when these cells overexpress ACE. Macrophages that overexpress ACE are more effective against tumours and infections. Neutrophils that overexpress ACE have an increased production of superoxide, which increases their ability to kill bacteria. These effects are due to increased ACE activity but are independent of angiotensin II. ACE also affects the display of major histocompatibility complex (MHC) class I and MHC class II peptides, potentially by enzymatically trimming these peptides. Understanding how ACE expression and activity affect myeloid cells may hold great promise for therapeutic manipulation, including the treatment of both infection and malignancy.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

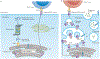

References
-
- Skeggs LT Jr Discovery of the two angiotensin peptides and the angiotensin converting enzyme. Hypertension 21, 259–260 (1993). - PubMed
-
- Metzger R et al. Heterogeneous distribution of angiotensin I-converting enzyme (CD143) in the human and rat vascular systems: vessel, organ and species specificity. Microvasc. Res 81, 206–215 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

