Lipodystrophic syndromes due to LMNA mutations: recent developments on biomolecular aspects, pathophysiological hypotheses and therapeutic perspectives
- PMID: 29578370
- PMCID: PMC5973242
- DOI: 10.1080/19491034.2018.1456217
Lipodystrophic syndromes due to LMNA mutations: recent developments on biomolecular aspects, pathophysiological hypotheses and therapeutic perspectives
Abstract
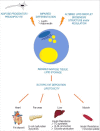
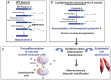
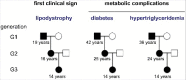
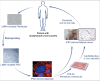
Mutations in LMNA, encoding A-type lamins, are responsible for laminopathies including muscular dystrophies, lipodystrophies, and premature ageing syndromes. LMNA mutations have been shown to alter nuclear structure and stiffness, binding to partners at the nuclear envelope or within the nucleoplasm, gene expression and/or prelamin A maturation. LMNA-associated lipodystrophic features, combining generalized or partial fat atrophy and metabolic alterations associated with insulin resistance, could result from altered adipocyte differentiation or from altered fat structure. Recent studies shed some light on how pathogenic A-type lamin variants could trigger lipodystrophy, metabolic complications, and precocious cardiovascular events. Alterations in adipose tissue extracellular matrix and TGF-beta signaling could initiate metabolic inflexibility. Premature senescence of vascular cells could contribute to cardiovascular complications. In affected families, metabolic alterations occur at an earlier age across generations, which could result from epigenetic deregulation induced by LMNA mutations. Novel cellular models recapitulating adipogenic developmental pathways provide scalable tools for disease modeling and therapeutic screening.
Keywords: Lamin A/C; adipose tissue; anticipation; differentiation; epigenetics; extracellular matrix; induced pluripotent stem cells; lipodystrophy; metreleptin; senescence.
Figures


References
-
- Cuthbertson DJ, Steele T, Wilding JP, Halford JC, Harrold JA, Hamer M, Karpe F. What have human experimental overfeeding studies taught us about adipose tissue expansion and susceptibility to obesity and metabolic complications? Int J Obes (Lond). 2017;41(6):853–865. - PubMed
-
- Stefan N, Schick F, Haring HU. Causes, Characteristics, and Consequences of Metabolically Unhealthy Normal Weight in Humans. Cell Metab. 2017;26(2):292–300. - PubMed
-
- Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, et al. . Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402(6764):880–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
