Clinical Pharmacokinetics of Sulfobutylether-β-Cyclodextrin in Patients With Varying Degrees of Renal Impairment
- PMID: 29578585
- PMCID: PMC6718009
- DOI: 10.1002/jcph.1077
Clinical Pharmacokinetics of Sulfobutylether-β-Cyclodextrin in Patients With Varying Degrees of Renal Impairment
Abstract
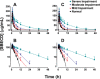
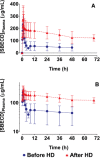
Delafloxacin, a fluoroquinolone, has activity against Gram-positive organisms including methicillin-resistant S aureus and fluoroquinolone-susceptible and -resistant Gram-negative organisms. The intravenous formulation of delafloxacin contains the excipient sulfobutylether-β-cyclodextrin (SBECD), which is eliminated by renal filtration. This study examined the pharmacokinetics and safety of SBECD after single intravenous (IV) infusions in subjects with renal impairment. The study was an open-label, parallel-group, crossover study in subjects with normal renal function or mild, moderate, or severe renal impairment, and those with end-stage renal disease undergoing hemodialysis. Subjects received 300 mg delafloxacin IV or placebo IV, containing 2400 mg SBECD, in 2 periods separated by ≥14-day washouts. SBECD total clearance decreased with decreasing renal function, with a corresponding increase in area under the concentration-time curve (AUC0-∞ ). After IV delafloxacin 300 mg administration, SBECD mean total clearance was 6.28 and 1.24 L/h, mean AUC0-∞ was 387 and 2130 h·μg/mL, and mean renal clearance was 5.36 and 1.14 L/h in normal and severe renal subjects, respectively. Similar values were obtained after IV placebo administration. In subjects with end-stage renal disease, delafloxacin 300 mg IV produced mean SBECD AUC0-48 values of 2715 and 7861 h·μg/mL when dosed before and after hemodialysis, respectively. Total SBECD clearance exhibited linear relationships to estimated glomerular filtration rate and creatinine clearance. Single doses of IV delafloxacin 300 mg and IV placebo were well tolerated in all groups. In conclusion, decreasing renal function causes reduced SBECD clearance and increased exposures, but SBECD continues to exhibit a good safety and tolerability profile in IV formulations.
Keywords: Delafloxacin; Hemodialysis; Pharmacokinetics; Renal Dysfunction; Sulfobutylether-β-cyclodextrin.
© 2018, The American College of Clinical Pharmacology.
Figures



References
-
- Delafloxacin Prescribing Information . https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208610s000,208.... Accessed August 18, 2017.
-
- O'Riordan W, McManus A, Teras J, et al. A global phase 3 study of delafloxacin (DLX) compared to vancomycin/aztreonam (VAN/AZ) in patients with acute bactyerial skin and skin structure infections (ABSSSI). Melinta Therapeutics Web Site. http://melinta.com/wp-content/uploads/2016/10/IDWEEK-1347-Baxdela-vs-VAN.... Accessed August 18, 2017.
-
- Beasley R, Oguchi G, Liang S, Lawrence L, Cammarata S. Delafloxacin (DLX) is effective and well‐tolerated in treatment of patients with renal impairment with acute bacterial skin and skin structure infections (ABSSSI) versus vancomycin/aztreonam (VAN/AZ). www.Melinta.com. http://melinta.com/wp-content/uploads/2017/04/ECCMID-2017-Renal.pdf. Accessed August 18, 2017.
-
- Study to compare delafloxacin to moxifloxacin for the treatment of adults with community‐acquired bacterial pneumonia. www.clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT02679573?term=delafloxacin&rank=3. Accessed August 18, 2017.
-
- Captisol® dosing in clinical and commercial products. Ligand Technology. www.Captisol.com. http://www.captisol.com/. Accessed August 18, 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

