ATP-Linked Chimeric Nucleotide as a Specific Luminescence Reporter of Deoxyuridine Triphosphatase
- PMID: 29578692
- PMCID: PMC6484856
- DOI: 10.1021/acs.bioconjchem.8b00124
ATP-Linked Chimeric Nucleotide as a Specific Luminescence Reporter of Deoxyuridine Triphosphatase
Abstract
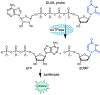
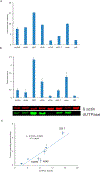
Nucleotide surveillance enzymes play important roles in human health, by monitoring damaged monomers in the nucleotide pool and deactivating them before they are incorporated into chromosomal DNA or disrupt nucleotide metabolism. In particular, deamination of cytosine, leading to uracil in DNA and in the nucleotide pool, can be deleterious, causing DNA damage. The enzyme deoxyuridine triphosphatase (dUTPase) is currently under study as a therapeutic and prognostic target for cancer. Measuring the activity of this enzyme is important both in basic research and in clinical applications involving this pathway, but current methods are nonselective, detecting pyrophosphate, which is produced by many enzymes. Here we describe the design and synthesis of a dUTPase enzyme-specific chimeric dinucleotide (DUAL) that replaces the pyrophosphate leaving group of the native substrate with ATP, enabling sensitive detection via luciferase luminescence signaling. The DUAL probe functions sensitively and selectively to quantify enzyme activities in vitro and in cell lysates. We further report the first measurements of dUTPase activities in eight different cell lines, which are found to vary by a factor of 7-fold. We expect that the new probe can be of considerable utility in research involving this clinically significant enzyme.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures

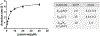
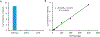

References
-
- Lindahl T (1993) Instability and decay of the primary structure of DNA. Nature 362, 709–715. - PubMed
-
- Brynolf K, Eliasson R, and Reichard P (1978) Formation of Okazaki fragments in polyoma DNA synthesis caused by misincorporation of uracil. Cell 13, 573–580. - PubMed
-
- Andersen S, Heine T, Sneve R, König I, Krokan HE, Epe B, and Nilsen H (2005) Incorporation of dUMP into DNA is a major source of spontaneous DNA damage, while excision of uracil is not required for cytotoxicity of fluoropyrimidines in mouse embryonic fibroblasts. Carcinogenesis 26, 547–555. - PubMed
-
- Friedberg E, Walker G, Siede W, Wood R, Schultz R, and Ellenberger T (1995) DNA Repair and Mutagenesis American Society for Microbiology Press, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

